Review Article
Antineoplastic Therapies related Cardiotoxicity
1Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan 430060, PR China
2Cardiovascular Research Institute of Wuhan University, Wuhan 430060, PR China
3Hubei Key Laboratory of Cardiology, Wuhan 430060, PR China
*Corresponding author: Zhou-Yan Bian, Department of Cardiology, Renmin Hospital of Wuhan University, Cardiovascular Research Institute of Wuhan University, Hubei Key Laboratory of Cardiology, Wuhan 430060, PR China, Tel/Fax: 86-27-88041911-89026, E-mail: zhouyanbian@whu.edu.cn
Received: August 12, 2019 Accepted: August 27, 2019 Published: September 3, 2019
Citation: Fu L, Bian Z-Y. Antineoplastic Therapies related Cardiotoxicity. Madridge J Intern Emerg Med. 2019; 3(2): 133-140. doi: 10.18689/mjiem-1000131
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Antineoplastic therapies related cardiotoxicity has become a significant concern for the long-term survival of cancer patients going through such treatments. Thus knowing the existing types of therapies that causes cardiotoxicity adverse effects as well as exploring their underlying mechanisms is of great clinical importance. In this article, we have made a review on the main types of cardiotoxicity-related antineoplastic therapies, which could be divided into traditional chemotherapy, targeted therapy, radiotherapy and immune therapy; and later we discussed on the possible mechanism of each of them. Besides, the monitoring and inspection methods which consists mainly of the echocardiography, MRI as well as some biomarkers, various as the methods, right now there however doesnʼt exist any standard or criterion for the early and timely detection of the cardiotoxicity effects for these patients. Furthermore, next we made a summary on the protection or prevention of the heart for patients treated or going to be treated with antineoplastic therapies, mainly based on the chemotherapy. Lastly, we made a discussion on the problems and challenges we are facing and drew a prospect of the future on cardio-oncology.
Keywords: Antineoplastic Therapy; Cardiotoxicity; Cancer patients.
Introduction
With the enhancement of the early-stage diagnosis technology, the rate of tumor diagnosis has been increasing year by year. With the maturity and wide application of chemotherapy and radiotherapy, the survival rate of cancer patients has been greatly improved. However, the side effects brought by these antineoplastic therapies have also emerged. Among them, cardiac toxicity directly affects the survival and long-term prognosis of patients, which makes it an essential aspect to take into consider when treating patients. After the occurrence of myocardial infraction (MI) it will further develop into refractory heart failure or fatal arrhythmias, which becomes a major cause of death among these patients [1]. Early detection and intervention of this process will be playing positive roles in the accurate prognosis as well as the decreased mortality of patients. Therefore, the cardiotoxicity caused by antineoplastic therapies and its possible mechanism, evaluation methods and treatment methods are worth exploring. In this review, we aim to: 1) make a summary of the antineoplastic therapies related cardiotoxicity and the mechanism; 2) summarize the detection as well as diagnosis methods of it; 3) summarize the novel prevention treatment of cardiotoxicity; 4) discuss and make a prospect on the future research directions.
Cardiotoxicity related Antineoplastic therapies and the mechanism
Traditional chemotherapy
Anthracyclines: Anthracyclines are highly effective antibiotics used against hematopoietic tumors and solid tumors, which have multiple cellular and subcellular targets, and thus induce cardiotoxicity. The main cardiotoxicity mechanism of anthracyclines is characterized by two main aspects, the first is that the reactive oxygen species(ROS) produced by anthracyclines through enzymatically conversion as well as reaction with the iron ions would produce highly reactive hydroxyl radicals, which in turn cause intracellular damage of the DNA, protein and lipids [2,3]. Whatʼs more, ROS stabilize p53, which consecutively initiates senescence and apoptotic cell death [4]. In mitochondria, anthracyclines through increased ROS formation promote DNA damage and opening of the mitochondrial permeability transition pore, which, in turn, results in collapse of the mitochondrial membrane potential, disruption of the outer mitochondrial membrane, release of cytochrome C into the cytosol and the initiation of cell death [5]. Besides, recent studies have shown that anthracyclines can also directly damage the myocardium by inhibiting topoisomerase. The main target of anthracyclines is topoisomerase IIα, which is highly expressed in tumors due to the active metabolism of tumor cells. Adult cardiomyocytes express only the topoisomerase IIβ isoenzyme. These structurally as well as catalytic-mechanically similar enzymes got damaged by anthracyclines at the same time [6]. Moreover, interference of anthracyclines with calcium channels increases intracellular calcium levels and induces calcium overload, which activates various proteases for further damage [7,8]. As for the vasculature, endothelial NO synthase (eNOS) activity is reduced, whereas cytosolic calcium is increased in smooth muscle cells, making the endothelial dysfunction patients more vulnerable to develop into heart failure [9,10]. Cardiomyopathy caused by anthracyclines is mostly irreversible and dose-dependent because its stimulation promotes the progression of cardiomyocytes from vacuolar swelling to fibrosis and eventual cell death, and, peak drug levels and associated toxicity are reduced possibly by prolonged infusion protocols [11].
Cyclophosphamide: Cyclophosphamide is an alkylation agent functioning on DNA. Cardiac toxicity of cyclophosphamide is relatively rare, which mainly occurs when overdosed (more than 140 mg/kg) before bone marrow transplantation [12]. The results of the French Pulmonary Hypertension Network showed that some chemotherapy treatment could lead to pulmonary artery occlusive diseases [13]. Among them, cyclophosphamide (43%), mitomycin C (24.3%) and cisplatin (21.6%) were most correlated with pulmonary artery thrombosis [13]. The formation of pulmonary artery thrombosis occurred one year after the beginning of chemotherapy. Pulmonary hypertension induced by cyclophosphamide is related to endothelial injury induced by oxidative stress [14].
Targeted therapy
Trastuzumab: Trastuzumab is a monoclonal antibody that inhibits the tyrosine protein kinase erbB-2 and erbB-3 of the receptor [15]. It specifically influences the extracellular site of the human epidermal growth factor receptor-2 (HER2). Both erbB-2 and erbB-3 are expressed in tumor cells, erbB-2, however, is also expressed in cardiomyocytes. Animal experiments have confirmed that the deletion of erbB-2 in cardiomyocytes will lead to dilated cardiomyopathy, confirming its important role in the proliferating as well as functioning in myocytes [16].
Studies have shown that patients treated with trastuzumab alone have a lower incidence of heart disease than those treated with anthracyclines [17]. When trastuzumab is combined with other antimetabolic drugs and alkylating agents for gastric cancer treatment, the incidence of cardiac insufficiency and heart failure were 5% and <1% respectively [18]. Pre-treatment of anthracyclines or combination treatment will add risk to the cardiotoxity of trastuzumab. Bowles et al. [19] showed that the incidence of cardiac insufficiency and/or heart failure in combination with anthracyclines and trastuzumab for one and five years was 6.2% and 20.1% respectively. Slamon et al. confirmed that among patients receiving anthracyclines, cyclophosphamide and trastuzumab, the incidence of NYHAIII and IV grade cardiac insufficiency was 27%; among those receiving anthracyclines or cyclophosphamide, it was 8%; and among those receiving paclitaxel and trastuzumab, it was 13%; while it was only 1% among patients who received paclitaxel alone [17].
Unlike anthracyclines, the typical cardiotoxicity reaction of trastuzumab usually occurs during the administration. Generally speaking, the cardiotoxicity of trastuzumab is non-dose-dependent: after discontinuation of trastuzumab and/or anti-heart-failure treatment, the associated left ventricular dysfunction and heart failure are usually reversible. This is because the mechanism of HER2 antibody-induced cardiotoxicity involves structural and functional changes in contractile proteins and mitochondria, but seldom leads to cell death [20].
Dashatinib: Dashatinib is an oral tyrosine kinase inhibitor for the first-line treatment of patients with chronic myeloid leukemia and acute lymphocytic leukemia as well as solid tumors such as prostate, ovarian and breast tumors. Dashatinib can cause endothelial cell damage, oxidative stress, and changes in the proportions of proliferation and inhibition of endothelial cells and arterial smooth muscle cells. These changes will lead to the increased susceptibility and pulmonary artery pressure. The French Pulmonary Hypertension Network showed that Dashatinib is also associated with pulmonary hypertension [13]. It has been shown that the clinical manifestations and organ functions of most patients have been improved after the discontinuation of Dashatinib. However, some patients died from hemodynamic complications.
Vascular endothelial growth factor (VEGF) signaling pathway inhibitors: VEGF inhibitors can cause reversible or irreversible cardiac remodeling, especially when combined with other chemotherapeutic drugs. This process may be associated with the inhibition of the positive effects of VEGF in cardiac vascular remodeling [21]. A clinical trial involving a large samples of breast cancer patients treated with anti-VEGF antibody bevacizumab after chemotherapy showed that, 2% of patients developed left ventricular dysfunction, and 1% of them developed heart failure (heart function III or IV) [22]. VEGF receptor tyrosine kinase inhibitors, such as sunitinib, acinetinib and pazopanib, cause heart failure in 3%-15% of patients and symptomatic heart failure in 1%-10% of patients [23-25]. Since the VEGF pathway and the related NO downstream signaling in the cardiovascular system got inhibited, eNOS- phosphorylation and reduced activity go along with increased vascular ROS levels which contribute to endothelial dysfunction, microvascular injury, vascular stiffness, and finally hypertension [26]. Likewise, VEGF inhibitors may promote thrombosis, eventually resulting in venous and arterial thromboembolic events [27]. However, whether to apply anticoagulation therapy is still under discussion, since VEGF targeted therapies also carry a relevant risk for bleeding [28,29], thus it requires individual decision making in case bleeding or thrombosis occurs. Timely control of blood pressure may reduce the risk of heart failure [30]. If cardiac insufficiency occurs, cardiac insufficiency may be reversed by rational and intensive anti-heart failure drugs.
Radiotherapy
It is estimated that more than 50% of modern cancer therapies include radiation therapy. Radiotherapy-induced heart disease (RIHD) occurs mainly in patients with Hodgkinʼs lymphoma, breast cancer (especially left breast), lung cancer, and other mediastinal malignancies (e.g. esophageal cancer) that require chemotherapy [31]. Radioactive heart injury mainly consists of: (1) Pericardial lesions: acute pericarditis, pericardial effusion and pericardial constriction [32]. (2) Heart muscle disease: high-dose radiotherapy heart inflammation, heart disease after chemotherapy [33,34]. (3) Coronary artery disease [35]. (4) Valvular disease and conduction abnormalities [36].
Radiotherapy first causes damage to the capillary endothelium of the heart, which in turn causes inflammation and activation of macrophages and monocytes, leading to tumor necrosis factor, monocyte chemotactic factor, interleukin 1,6,8, transforming growth factor β, insulin-like growth factor and platelet differentiation growth factor [37]. Damage to the endothelium also causes activation of the coagulation mechanism, causing the cellulose to deposit. The above changes activate the proliferation of endothelial cells and eventually lead to blockage of the microcirculation [38]. Activation of matrix metalloproteinases induces degradation of the endothelial basement membrane, which in turn causes pro-inflammatory cells to aggregate at the damaged tissue. In the advanced stage, the gradual occlusion of the microcirculation and the formation of thrombus cause ischemia and necrosis of the cells, which in turn leads to the replacement of the myocardium by fibrous tissue and may lead to cardiac insufficiency or even heart failure [39,40]. Moreover, chronic oxidative stress damage with persistent free radical generation also increases the occurrence of advanced atherosclerotic disease [31]. Animal studies on myocardium and pericardial tissue have shown an increase in the number of inflammatory cells and fibroblasts after radiotherapy and a significant increase in extracellular matrix (including collagen, glycoprotein and fibronectin) [39].
Another important factor that should not be overlooked is the lung damage caused by radiotherapy. Radiotherapy not only can cause fibrosis of the lungs and lead to pulmonary interstitial fibrosis; but it can also cause endothelial damage and inflammation in the microcirculation of the lungs. Both of these cause an increase in lung resistance and pulmonary wedge pressure and eventually cause or worsen the remodeling of the right ventricle [31].
Cardiotoxicity caused by chemotherapy can occur after a few weeks; however, RIHD usually occurs much later – more often after 5-10 years of the therapy [31,41]. Acute radiation reactions are usually subtle, so the monitoring is more difficult and clinically relevant [36]. When a patient develops cardiovascular symptoms after radiotherapy, his or her adverse cardiac reactions should be assessed immediately.
Immune therapy
Immunotherapy is another efficient way to improve the prognosis of patients with malignant tumors which has changed the treatment landscape of advanced cancers [42]. Immunoassay point inhibitors (ICI) are a class of immunoregulatory factors that affect the activity of T cells by binding with negative immunoregulatory factors on the surface of T cells or tumor cells. At present, cytotoxic T lymphocyte associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) and their ligand PD-L1 have been well studied. Unfortunately, unlike traditional chemotherapeutic drugs or molecular targeted drugs, ICIs can activate non-specific immunity and induce a wide spectrum of adverse events, known as the immune-related adverse events (irAEs) [43], among which the currently understood spectrum of cardiac pathology includes myocarditis, dilated cardiomyopathy, pericardial effusion, and arrhythmias, with autoimmune myocarditis being the best characterized to date [44-50]. Thyroiditis can lead to thyroid crisis, atrial fibrillation, ventricular arrhythmia and heart failure [51,52]. Although the exact pathophysiological mechanism is not yet fully understood, biopsies from affected organ systems have demonstrated lymphocytic infiltration, reflecting an autoimmune process [53].
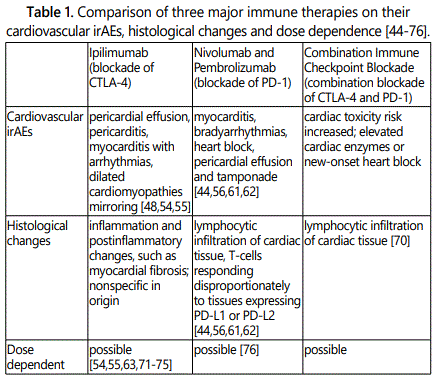
The immune therapy mainly functions by blocking CTLA-4 (Ipilimumab), blocking PD-1 (Nivolumab and Pembrolizumab) as well as the combination of the above two. Cardiovascular irAEs attributed to these drugs varies from pericardial effusion and pericarditis, to myocarditis and arrhythmias etc. [48,54,55] (Table 1), nevertheless, all three therapies are responsible for the exacerbation of heart failure, with the signs of dyspnea, edema, increased B type natriuretic peptide level, or decreased ejection fraction [44-76] which is of great clinical significance. Overall, irAEs attributed to CTLA-4 blockade are considered to be more severe than those associated with inhibition of PD-1 [65]. Whatʼs more, studies have shown that the cardiac toxicity effect of the above therapies is usually dose-dependent [66]. However, up to now, there are no relevant guidelines or consensus that clearly put forward for the use of immunoassay point inhibitors in the treatment of patients on how to carry out risk population screening, disease monitoring and effective treatment after the onset of the disease.
Monitoring and Inspection
Firstly, high-risk patients need to be identified and screened, including a careful assessment of baseline cardiovascular risk factors. Risk factors assessment includes asking for medical history and auxiliary examinations, such as imaging methods to assess baseline cardiac function before tumor treatment. In addition, cardiac biomarkers (natriuretic peptide or troponin) are critical for detecting subclinical cardiac abnormalities, which are also very valuable for the selection, assessment of indications for cardioprotective therapy, and deciding whether to increase the frequency of monitoring for asymptomatic left ventricular dysfunction or not.
Detection of the cardiotoxicity has gone through great revolution with the advancement of examination techniques. Traditional method like ECG is still being widely used clinically but owing to the lack of sensitivity and specificity, itʼs more often used as part of the routine check and might be able to seize the transient changes indicating cardiotoxicity. Echocardiography is known as the standard method for the evaluation of cardiac function. Cardiotoxicity is defined as a decrease in 3D echocardiography ejection fraction by 10% to a level below 50% [77], and echocardiography should be redone to confirm whether LVEF will keep dropping 2-3 weeks after the drop is first detected. Speckle tracking global longitudinal strain (GLS) imaging has shown higher specificity and sensitivity, lower intra- and inter-observer variability, and provides comparable results to magnetic resonance imaging (MRI) [78]. Echocardiographic three dimensional speckle tracking imaging (3D-STI) evaluation of the LV provides an understanding of the segmental impairment of LV wall and the possible process of LV impairment in lymphoma patients after anthracycline chemotherapy [79]. MRI remains the gold standard for the quantification of cardiac dimensions and ventricular function, but is often only used as a secondary option – mainly in patients with poor echocardiography windows or inconclusive results [80]. Special applications of cardiac magnetic resonance (CMR) to assess for cancer therapy–induced cardiac toxicity include the detection of subclinical LV dysfunction through novel methods of measuring myocardial strain, detection of microcirculatory dysfunction, identification of LV and LA fibrosis, and more sensitive detection of inflammation caused by immune checkpoint inhibitors, which plays a significant role in the non-invasive workup of cardiac toxicity from cancer therapies [81]. As for the cardiac biomarkers for detection of the cardiotoxicity, thereʼs no standard criterion currently, however, troponin and brain natriuretic peptide (BNP) levels are routinely measured in tumor patients receiving chemotherapy. Troponin elevation has been used to reflect the susceptibility of cardiomyopathy-inducing drugs, particularly anthracycline therapies [82-84]. Whatʼs more, the combined use of troponin measurement and echocardiography has been superior in the diagnosis of cardiotoxicity over echocardiography alone [78]. For patients undergo trastuzumab treatments, especially those who had been previously treated with anthracyclines, the elevation of troponin I can be used to recognize patients of cardiac dysfunction and those that are hard to restore their cardiac function. The sensitivity of highsensitivity troponin I combined with GLS examination for predicting the occurrence of heart failure in breast cancer patients after chemotherapy can reach 93% [85]. The exact definition of biomarker distribution and cut-off level still wait for future trials to assess. Currently, troponin should be assessed in patients before and during anthracycline therapy, during checkpoint inhibitor medication, and whenever ischemic heart disease is suspected [80].
All patients receiving potential cardiotoxicity chemotherapy should undergo a cardiac assessment during the follow-up period after the end of the treatment. The proper time to for these inspections should be individualized according to the underlying cardiovascular risks and specific cancer treatment regimens.
Protection and Prevention
Over the past few decades, the recognizing of neoplastic therapy related cardiac toxicity has shifted to treating as well as preventing. Reviewing on the current treatments as well as protective therapy to impede and alleviate the cardiotoxicity is of great clinical significant for the better prognosis of patients.
The most effective approach to minimize cardiotoxicity is early identification and early onset of a prophylactic treatment [86]. However, with the lack of efficient cardiac assessment standards for predicting the cardiotoxicity in advance, some prevention measures have to be taken for the better prognosis of patients. Firstly, limitation of the maximum dose of antracyclines is of great clinical significance [87], but due to the genetic variation as well as the compromise of effect-reducing when the dose decreased, individualized dose limitation needs to be explored. Studies show that minimizing anthracycline exposure, or when possible, avoiding anthracycline-based regimens in breast cancer patients, and in young and old populations who are more vulnerable to anthracycline cardiotoxic effect–should be taken into consideration [88-90]. Secondly, some less cardiotoxic anthracycline analogues could be used to alleviate the adverse effects. Epirubicin, idarubicin and mitoxantrone are analogues of anthracyclines that have been shown to be less cardiotoxic than conventional anthracyclines in preclinical and clinical studies [86]. Epirubicin cardiotoxicity occurs after higher doses than doxorubicin, but higher doses must be administered to achieve the same clinical response (90 mg/mq epirubicin=60 mg/mg doxorubicin). Idarubicin and mitoxantrone also showed a less cardiotoxic profile than doxorubicin in pre-clinical studies and animal models, clinical trials is needed for further confirm of this effect [87,91]. Thirdly, liposome encapsulation anthracyclines have been proved efficient in reducing the cardiotoxicity. Liposomal formulations are small enough (80–90 nm) to penetrate through the more fragile fenestrated microvasculature that characterizes solid tumors, resulting in preferential accumulation in tumors and minimal release in plasma and healthy tissues, voiding the high plasma levels of free doxorubicin, which is strongly associated with cardiac toxicity, as well as not compromising the tumoricidal efficacy [92-94].
As for the medical therapies: Firstly, several kinds of β-blockers are considered cardioprotective according to clinical as well as preclinical trials. Among them, Carvedilol, a non-cardioselective beta-blocker with antioxidant properties, has been shown to be a cardioprotective effective against anthracyclines toxicity both in vivo studies and in clinical trials [95-97], with the function of preventing the LVEF reduction. The exact mechanism by which carvedilol exerts its cardioprotective effect is unclear but it appears to be related to its antioxidant activity, rather than to its beta-blocking action when compared with other β-blockers [91,98]. The cardioprotective action of nebivolol, a selective β1 antagonist with nitric oxide-dependent vasodilatory properties, has been demonstrated a small clinical trial of breast cancer patients, the result showed no significant improvements of the LVEF or the BNP level, but patients of the treated group suffered comparably less LVEF reduction [99]. Secondly, since the renin angiotensin system (RAS) plays a crucial role in the development and progression of cardiotoxicity induced by anthracyclines, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are considered effective in the treatment of cardiotoxicity [100]. Valsartan, an angiotensin II receptor blocker, when administered together with anthracyclines, was able to prevent the increase of cardiac biomarkers such as ANP and BNP, the increase of tele-diastolic left ventricular diameter, and QTc interval [101]. The angiotensin II receptor blocker telmisartan, initiated 1 week before epirubicin in 25 patients with various solid tumors, was able to prevent significant reduction in myocardial deformation parameters and an increase in reactive oxygen species or in interleukin-6, not only because of its RAS blocking action, but also because of its anti-inflammatory and anti-oxidant properties [102]. Thirdly, aldosterone antagonists like spironolactone has been evaluated versus placebo in a recent randomized trial, pateints didnʼt show significant reductions in FEVS, and had a preserved diastolic function with no increase in troponin I and NT-proBNP after starting the drug one week before anthracycline-including chemotherapy [103]. Fourthly, known for their antioxidant effect, statins are also considered cardioprotective against anthracycline [104]. Whatʼs more, the protective effect of statins also seemed to present in patients already receiving statins for prevention of cardiovascular disease when chemotherapy was started [105].
Discussion and Prospect
With more patientsʼ life spans got prolonged thanks to the modern antineoplastic therapies, problems arise as the adverse effects of them have become more and more the concern clinically. At present, antineoplastic therapies and their cardiotoxicity effects have been better known with clinical practices, and the problems behind could be divided into: 1) Lack of the knowledge of the exact cardiotoxicity mechanism of each therapy; 2) Lack of a complete system or standard for the measurement of the cardiac function of patients going through antineoplastic therapies; 3) Lack of criteria for the detection of the cardiotoxicity effects before symptoms appear of the clinical biomarkers and other early index; 4) Lack of a standard prevention and treatment strategy specialized for these patients. For future studies and trials, all of the above problems await for solution and with a better knowledge of the potential mechanisms of the cardiotoxicity effects, more and more efficient treatment will be figured out.
As an emerging subspecialty, oncocardiology shows a complex and close relationship between neoplasia and cardiovascular disease. However, due to the specificity of tumors and cardiovascular diseases, there are many differences in clinical management strategies between patients with tumors and cardiovascular diseases. To this end, the collaboration between oncologists and cardiovascular doctors is becoming closer, and a safer and more effective chemotherapy regimen is being explored to establish a practical cardiotoxicity prevention and treatment system for a brighter prognosis of the patients.
Acknowledgment
This project was supported by the National Natural Science Foundation of China (81470402).
References
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013; 6(3): 606-619. doi: 10.1161/HHF.0b013e318291329a
- Minotti G, Ronchi R, Salvatorelli E, Menna P, Cairo G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for ironmediated cardiotoxicity of antitumor therapy. Cancer Res. 2001; 61(23): 8422-8428.
- Rochette L, Guenancia C, Gudjoncik A, et al. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. 2015; 36(6): 326-348. doi: 10.1016/j.tips.2015.03.005
- Park AM, Nagase H, Liu L, et al. Mechanism of anthracycline-mediated down-regulation of GATA4 in the heart. Cardiovasc Res. 2011; 90(1): 97-104. doi: 10.1093/cvr/cvq361
- Salimi A, Neshat MR, Naserzadeh P, Pourahmad J. Mitochondrial Permeability Transition Pore Sealing Agents and Antioxidants Protect Oxidative Stress and Mitochondrial Dysfunction Induced by Naproxen, Diclofenac and Celecoxib. Drug Res (Stuttg). 2019; doi: 10.1055/a-0866-9356
- Vejpongsa P, Yeh ET. Topoisomerase 2β: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther. 2014; 95(1): 45-52. doi: 10.1038/clpt.2013.201
- Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015; 131(22): 1981-1988. doi: 10.1161/CIRCULATIONAHA.114.013777
- Yeh ET, Chang HM. Oncocardiology-Past, Present, and Future: A Review. JAMA Cardiol. 2016; 1(9): 1066-1072. doi: 10.1001/jamacardio.2016.2132
- Gajalakshmi P, Priya MK, Pradeep T, et al. Breast cancer drugs dampen vascular functions by interfering with nitric oxide signaling in endothelium. Toxicol Appl Pharmacol. 2013; 269(2): 121-131. doi: 10.1016/j.taap.2013.03.011
- Finkelman BS, Putt M, Wang T, et al. Arginine-Nitric Oxide Metabolites and Cardiac Dysfunction in Patients with Breast Cancer. J Am Coll Cardiol. 2017; 70(2): 152-162. doi: 10.1016/j.jacc.2017.05.019
- Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982; 96(2): 133-139. doi: 10.7326/0003-4819-96-2-133
- Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991; 9(7): 1215-1223. doi: 10.1200/JCO.1991.9.7.1215
- Ranchoux B, Günther S, Quarck R, et al. Chemotherapy-induced pulmonary hypertension: role of alkylating agents. Am J Pathol. 2015; 185(2): 356-371. doi: 10.1016/j.ajpath.2014.10.021
- Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA Damage and Pulmonary Hypertension. Int J Mol Sci. 2016; 17(6): E990. doi: 10.3390/ijms17060990
- Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002; 8(5): 459-465. doi: 10.1038/nm0502-459
- Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res. 2004; 59: 1-12.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344(11): 783-792. doi: 10.1056/NEJM200103153441101
- Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015; 33(16): 1760-1769. doi: 10.1200/JCO.2014.60.1799
- Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012; 104(17): 1293-1305. doi: 10.1093/jnci/djs317
- Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med. 2013; 368(9): 876. doi: 10.1056/NEJMc1215887
- Toyota E, Warltier DC, Brock T, et al. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005; 112(14): 2108-2113. doi: 10.1161/CIRCULATIONAHA.104.526954
- Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013; 14(10): 933-942. doi: 10.1016/S1470-2045(13)70335-8
- Steingart RM, Bakris GL, Chen HX, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J. 2012. 163(2): 156-163. doi: 10.1016/j.ahj.2011.10.018
- Kim JH, Park I, Lee JL. Pazopanib versus sunitinib for the treatment of metastatic renal cell carcinoma patients with poor-risk features. Cancer Chemother Pharmacol. 2016; 78(2): 325-332. doi: 10.1007/s00280-016-3093-8
- Neves KB, Rios FJ, van der Mey L, et al. VEGFR (Vascular Endothelial Growth Factor Receptor) Inhibition Induces Cardiovascular Damage via Redox-Sensitive Processes. Hypertension. 2018; 71(4): 638-647. doi: 10.1161/HYPERTENSIONAHA.117.10490
- Advani A, Kelly DJ, Advani SL, et al. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A. 2007; 104(36): 14448-14453. doi: 10.1073/pnas.0703577104
- Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007; 99(16): 1232-1239. doi: 10.1093/jnci/djm086
- Totzeck M, Mincu RI, Rassaf T. Cardiovascular Adverse Events in Patients with Cancer treated with Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. J Am Heart Assoc. 2017; 6(8): e006278. doi: 10.1161/JAHA.117.006278
- Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: A meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018; 25(5): 482-494. doi: 10.1177/2047487318755193
- Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and Metabolic Implications of Novel Targeted Cancer Therapies: Focus on Kinase Inhibitors. J Am Coll Cardiol. 2015; 66(10): 1160-1178. doi: 10.1016/j.jacc.2015.07.025
- Tadic M, Cuspidi C, Hering D, Venneri L, Grozdic-Milojevic I. Radiotherapyinduced right ventricular remodelling: The missing piece of the puzzle. Arch Cardiovasc Dis. 2017; 110(2): 116-123. doi: 10.1016/j.acvd.2016.10.003
- Cuomo JR, Sharma GK, Conger PD, Weintraub NL. Novel concepts in radiation-induced cardiovascular disease. World J Cardiol. 2016; 8(9): 504-519. doi: 10.4330/wjc.v8.i9.504
- van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015; 175(6): 1007-1017.
- Azizova TV, Batistatou E, Grigorieva ES, et al. An Assessment of RadiationAssociated Risks of Mortality from Circulatory Disease in the Cohorts of Mayak and Sellafield Nuclear Workers. Radiat Res. 2018; 189(4): 371-388. doi: 10.1667/RR14468.1
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation DoseResponse Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol. 2016; 34(3): 235-243. doi: 10.1200/JCO.2015.63.4444
- Taylor C, Correa C, Duane FK, et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol. 2017; 35(15): 1641-1649. doi: 10.1200/JCO.2016.72.0722
- Burger A, Löffler H, Bamberg M, Rodemann HP. Molecular and cellular basis of radiation fibrosis. Int J Radiat Biol. 1998; 73(4): 401-408.
- Sardaro A, Petruzzelli MF, DʼErrico MP, Grimaldi L, Pili G, Portaluri M. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol. 2012; 103(2): 133-142. doi: 10.1016/j.radonc.2012.02.008
- Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015; 5: 39. doi: 10.3389/fonc.2015.00039
- Chello M, Mastroroberto P, Romano R, Zofrea S, Bevacqua I, Marchese AR. Changes in the proportion of types I and III collagen in the left ventricular wall of patients with post-irradiative pericarditis. Cardiovasc Surg. 1996; 4(2): 222-226. doi: 10.1016/0967-2109(96)82320-9
- Formenti SC, Lymberis SC, DeWyngaert JK. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med. 2013; 368(26): 2525. doi: 10.1056/NEJMc1304601
- Tajiri K, Ieda M. Cardiac Complications in Immune Checkpoint Inhibition Therapy. Front Cardiovasc Med. 2019; 6: 3. doi: 10.3389/fcvm.2019.00003
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015; 373(19): 1803-1813. doi: 10.1056/NEJMoa1510665
- Behling J, Kaes J, Münzel T, Grabbe S, Loquai C. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res. 2017; 27(2): 155-158. doi: 10.1097/CMR.0000000000000314
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018; 71(16): 1755-1764. doi: 10.1016/j.jacc.2018.02.037
- Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun. 2013; 45: 90-96. doi: 10.1016/j.jaut.2013.05.004
- Norwood TG, Westbrook BC, Johnson DB, et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017; 5(1): 91. doi: 10.1186/s40425-017-0296-4
- Reuben A, Petaccia de Macedo M, McQuade J, et al. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology. 2017; 6(12): e1361097. doi: 10.1080/2162402X.2017.1361097
- Zhang L, Jones-OʼConnor M, Awadalla M, et al. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr Treat Options Cardiovasc Med. 2019; 21(7): 32. doi: 10.1007/s11936-019-0731-6
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018; 19(12): 1579-1589. doi: 10.1016/S1470-2045(18)30608-9
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011; 480(7378): 480-489.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366(26): 2443-2454. doi: 10.1056/NEJMoa1200690
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018; 378(2): 158-168. doi: 10.1056/NEJMra1703481
- Geisler BP, Raad RA, Esaian D, Sharon E, Schwartz DR. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer. 2015; 3: 4. doi: 10.1186/s40425-015-0048-2
- Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med. 2015; 2015: 794842. doi: 10.1155/2015/794842
- Tay RY, Blackley E, McLean C, et al. Successful use of equine antithymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer. 2017; 117(7): 921-924. doi: 10.1038/bjc.2017.253
- Läubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015; 3: 11. doi: 10.1186/s40425-015-0057-1
- Frigeri M, Meyer P, Banfi C, et al. Immune Checkpoint Inhibitor-Associated Myocarditis: A New Challenge for Cardiologists. Can J Cardiol. 2018; 34(1): 92.e1-92.e3. doi: 10.1016/j.cjca.2017.09.025
- Tadokoro T, Keshino E, Makiyama A, et al. Acute Lymphocytic Myocarditis with Anti-PD-1 Antibody Nivolumab. Circ Heart Fail. 2016; 9(10): e003514. doi: 10.1161/CIRCHEARTFAILURE.116.003514
- Nesfeder J, Elsensohn AN, Thind M, Lennon J, Domsky S. Pericardial effusion with tamponade physiology induced by nivolumab. Int J Cardiol. 2016; 222: 613-614. doi: 10.1016/j.ijcard.2016.08.023
- Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016; 60: 210-225. doi: 10.1016/j.ejca.2016.02.024
- Matson DR, Accola MA, Rehrauer WM, Corliss RF. Fatal Myocarditis Following Treatment with the PD-1 Inhibitor Nivolumab. J Forensic Sci. 2018; 63(3): 954-957. doi: 10.1111/1556-4029.13633
- Heinzerling L, Ott PA, Hodi FS, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016; 4: 50. doi: 10.1186/s40425-016-0152-y
- Semper H, Muehlberg F, Schulz-Menger J, Allewelt M, Grohé C. Druginduced myocarditis after nivolumab treatment in a patient with PDL1-negative squamous cell carcinoma of the lung. Lung Cancer. 2016; 99: 117-119. doi: 10.1016/j.lungcan.2016.06.025
- Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014; 6(230): 230ra45. doi: 10.1126/scitranslmed.3008002
- Tajiri K, Ieda M. Cardiac Complications in Immune Checkpoint Inhibition Therapy. Front Cardiovasc Med. 2019; 6: 3. doi: 10.3389/fcvm.2019.00003
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016; 387(10027): 1540-1550. doi: 10.1016/S0140-6736(15)01281-7
- Wang J, Okazaki IM, Yoshida T, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010; 22(6): 443-452. doi: 10.1093/intimm/dxq026
- Baban B, Liu JY, Qin X, Weintraub NL, Mozaffari MS. Upregulation of Programmed Death-1 and Its Ligand in Cardiac Injury Models: Interaction with GADD153. PLoS One. 2015; 10(4): e0124059. doi: 10.1371/journal. pone.0124059
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016. 375(18): 1749-1755. doi: 10.1056/NEJMoa1609214
- Samara Y, Yu CL, Dasanu CA. Acute autoimmune myocarditis and hepatitis due to ipilimumab monotherapy for malignant melanoma. J Oncol Pharm Pract. 2019; 25(4): 966-968. doi: 10.1177/1078155218755868
- Dasanu CA, Jen T, Skulski R. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune monoarthritis induced by ipilimumab use for metastatic melanoma. J Oncol Pharm Pract. 2017. 23(3): 231-234. doi: 10.1177/1078155216635853
- Copur MS, Obermiller A. Ipilimumab plus dacarbazine in melanoma. N Engl J Med. 2011; 365(13): 1256-1257. doi: 10.1056/NEJMc1108661
- Hodi FS, OʼDay SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363(8): 711-723. doi: 10.1056/NEJMoa1003466
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015; 16(5): 522-530. doi: 10.1016/S1470-2045(15)70122-1
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013; 369(2): 134-144. doi: 10.1056/NEJMoa1305133
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016; 37(36): 2768-2801. doi: 10.1093/eurheartj/ehw211
- Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014; 63(25 Pt A): 2751-2768. doi: 10.1016/j.jacc.2014.01.073
- Xu Y, Shi J, Zhao R, et al. Anthracycline induced inconsistent left ventricular segmental systolic function variation in patients with lymphoma detected by three-dimensional speckle tracking imaging. Int J Cardiovasc Imaging. 2019; 35(5): 771-779. doi: 10.1007/s10554-018-1510-2
- Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019; 280: 163-175. doi: 10.1016/j.ijcard.2019.01.038
- Soufer A, Baldassarre LA. The Role of Cardiac Magnetic Resonance Imaging to Detect Cardiac Toxicity from Cancer Therapeutics. Curr Treat Options Cardiovasc Med. 2019; 21(6): 28. doi: 10.1007/s11936-019-0732-5
- Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014; 63(8): 809-816. doi: 10.1016/j.jacc.2013.10.061
- Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol. 2000; 36(2): 517-522. doi: 10.1016/s0735-1097(00)00748-8
- Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010; 28(25): 3910-3916. doi: 10.1200/JCO.2009.27.3615
- Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012; 5(5): 596-603. doi: 10.1161/CIRCIMAGING.112.973321
- Cardinale D, Stivala F, Cipolla CM. Oncologic therapies associated with cardiac toxicities: how to minimize the risks. Expert Rev Anticancer Ther. 2019; 19(5): 359-374. doi: 10.1080/14737140.2019.1596804
- Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J Am Coll Cardiol. 2017; 70(20): 2552-2565. doi: 10.1016/j.jacc.2017.09.1095
- Caron J, Nohria A. Cardiac Toxicity from Breast Cancer Treatment: Can We Avoid This? Curr Oncol Rep. 2018; 20(8): 61. doi: 10.1007/s11912-018-0710-1
- Mehta LS, Watson KE, Barac A, et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation. 2018; 137(8): e30-e66.
- Zarifa A, Albittar A, Kim PY, et al. Cardiac toxicities of anticancer treatments: chemotherapy, targeted therapy and immunotherapy. Curr Opin Cardiol. 2019; 34(4): 441-450. doi: 10.1097/HCO.0000000000000641
- Cardinale D, Biasillo G, Cipolla CM. Curing Cancer, Saving the Heart: A Challenge That Cardioncology Should Not Miss. Curr Cardiol Rep. 2016; 18(6): 51. doi: 10.1007/s11886-016-0731-z
- Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014; 64(9): 938-945. doi: 10.1016/j.jacc.2014.06.1167
- Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat. 2016; 29: 90-106. doi: 10.1016/j.drup.2016.10.003
- Menna P, Salvatorelli E. Primary Prevention Strategies for Anthracycline Cardiotoxicity: A Brief Overview. Chemotherapy. 2017; 62(3): 159-168. doi: 10.1159/000455823
- Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006; 48(11): 2258-2262. doi: 10.1016/j.jacc.2006.07.052
- Elitok A, Oz F, Cizgici AY, et al. Effect of carvedilol on silent anthracyclineinduced cardiotoxicity assessed by strain imaging: A prospective randomized controlled study with six-month follow-up. Cardiol J. 2014; 21(5): 509-515. doi: 10.5603/CJ.a2013.0150
- Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J Am Coll Cardiol. 2018; 71(20): 2281-2290. doi: 10.1016/j.jacc.2018.02.049
- Oliveira PJ, Bjork JA, Santos MS, et al. Carvedilol-mediated antioxidant rotection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol. 2004; 200(2): 159-168. doi: 10.1016/j.taap.2004.04.005
- Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol. 2013; 167(5): 2306-2310. doi: 10.1016/j.ijcard.2012.06.023
- Bloom MW, Hamo CE, Cardinale D, et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circ Heart Fail. 2016; 9(1): e002661. doi: 10.1161/CIRCHEARTFAILURE.115.002661
- Nakamae H, Tsumura K, Terada Y, et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer. 2005; 104(11): 2492-2498. doi: 10.1002/cncr.21478
- Cadeddu C, Piras A, Mantovani G, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. 2010; 160(3): 487.e1-e7. doi: 10.1016/j.ahj.2010.05.037
- Akpek M, Ozdogru I, Sahin O, et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail. 2015; 17(1): 81-89. doi: 10.1002/ejhf.196
- Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2011; 58(9): 988-989. doi: 10.1016/j.jacc.2011.05.025
- Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012; 60(23): 2384-2390.


