Research Article
Bivalirudin Efficiency to improve the Coronary flow of Obstructive Coronary Artery Patients
1Professor and HOU-IV, Department of Cardiology, Specialty Block Second Floor, Nizam’s Institute of Medical Sciences (NIMS), Hyderabad, India
2PhD student, Department of Cardiology, Specialty Block Second Floor, Nizam’s Institute of Medical Sciences (NIMS), Pujagutta, Hyderabad, India
3Consultant Cardiologist, Department of Cardiology, KIMS hospital, Door No 4-120/A, North, Bypass Road, Mukthinuthalapadu, Ongole AP, India
4Lab technician (Cardiovascular Technology), Department of Cardiology, Specialty Block Second Floor, Nizam’s Institute of Medical Sciences
(NIMS), Pujagutta, Hyderabad, India
*Corresponding author: Maddury Jyotsna, Professor and HOU-IV, Department of Cardiology, Specialty Block Second Floor, Nizam’s Institute of Medical Sciences (NIMS), Hyderabad, India, Ph: +91-9491073627, E-mail: mail2jyotsna@rediffmail.com
Received: January 21, 2019 Accepted: January 23, 2019 Published: January 29, 2019
Citation: Jyotsna M, Indrani G, Babu KS and Kumar RNV N. Bivalirudin Efficiency to improve the Coronary flow of Obstructive Coronary Artery Patients. Madridge J Intern Emerg Med. 2019; 3(1): 110-113. doi: 10.18689/mjiem-1000125
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Bivalirudin is a direct reversible inhibitor of thrombin. The synthetic 20 amino acid peptide is an active substance. As it facilitates to improve the coronary flow after PCI, we want to observe whether the Bivalirudin can improve the coronary flow before PCI.
Methodology: We prospectively recruited patients of the acute coronary syndrome (ACS) excluding ST elevation MI with single significant coronary stenosis undergoing percutaneous coronary intervention (PCI). Immediately after basal culprit vessel angiogram in an appropriate view, 0.75 mg/kg of Bivalirudin intravenous bolus was started followed by a 1.75 kg/mg/hour infusion. Before doing any balloon dilatation or any other treatment to culprit lesion, the angiogram was repeated for the same previous view of the culprit’s vessel. TIMI frame count (TFC) was taken as an indicator of coronary flow. TFC of culprit vessel was noted from basal and after Bivalirudin angiogram. Bivalirudin infusion was continued throughout the PCI procedure.
Results: In 50 eligible Acute Coronary Syndrome patients with the mean age of 54.4 ± 14.72 yrs were recruited in this study. The culprit lesion was in LAD 23 (46%), in LCX in 15(30%) and RCA lesion in 12(24%) of patients. 40% of patients had diabetes. The Mean TFC before Bivalirudin was 16.92 ± 6.2 vs. 11.4 ± 3.8 after Bivalirudin which was statistically significant (p=<0.0001).
Conclusion: Bivalirudin decrease the TIMI frame count (TFC) in acute coronary syndrome patients, before treating the culprit vessel angioplasty. As TFC depends on the coronary flow, we conclude that Bivalirudin improves coronary flow.
Keywords: Bivalirudin; TIMI (Thrombolysis in Myocardial Infarction) frame count (TFC); Percutaneous Coronary Intervention (PCI); Acute coronary syndrome (ACS); Cardiology”
Introduction
Bivalirudin is a direct thrombin inhibitor (DTI) polypeptide that reversibly inhibits thrombin by specifically binding both to the catalytic site and to the anion-binding exosite of circulating and clot-bound thrombin. The action of bivalirudin is reversible because thrombin will slowly cleave the thrombin-bivalirudin bond which recovers the active site of thrombin. This transient binding and self-reversing property of bivalirudin result in a biologic half-life of approximately 25 minutes. Bivalirudin offers more benefits compared to heparin, it is less dependent on renal function and has a lower anaphylaxis incidence. Bivalirudin does not activate circulating platelets, in contrast to heparin. It also has a platelet inhibitory effect via inhibition of thrombin-mediated platelet aggregation. It is also not inactivated by platelet release reaction components (e.g., Platelet factor 4). As a result, bivalirudin shows anticoagulation levels that are less variable than heparin [1].
Bivalirudin is indicated as an anticoagulant in percutaneous coronary intervention (PCI) patients with acute coronary syndrome (ACS). It is also indicated for use for those at risk of having heparin-induced thrombocytopenia and as an adjuvant along with IIb/IIIa (GPI) inhibitor [1].
Heparin has been the main source of anticoagulation during PCI for decades in patients with angina and ACS [2]. However, the HEAT PPCI trial [3] and the BRIGHT trial [4] both are favoring the use of bivalirudin instead of heparin. Most of the studies seen to immediate and long term outcomes after bivalirudin usage in these ACS patients, but none of the studies, as far as our knowledge, seen the improvement of coronary blood flow without treating the culprit lesion mechanically.
Material and Methods
Acute coronary syndrome (ACS) excluding ST elevation MI, with single significant coronary stenosis that was undergoing percutaneous coronary intervention (PCI) patients, was recruited prospectively in the study.
The first angiogram of the culprit vessel was taken in different views. One appropriate view which is showing culprit lesion was selected, and again that view was repeated with prolonged cine angiogram, including the whole length of the coronary artery with the myocardial blush. Then, Bivalirudin 0.75 mg/kg as intravenous bolus was started immediately, followed by an infusion of 1.75 kg/mg/hour. Then the angiogram of the culprit vessel was repeated in the same previous view, 3 minutes after starting the infusion. The TFC was taken as a coronary flow indicator. The TIMI (Thrombolysis in Myocardial Infarction) frame count (TFC) of the culprit vessel (up to the last segment of that particular vessel) was recorded from basal and after Bivalirudin infusion. The improper engagement of guide catheter or different sizes guides for basal and subsequent angioplasty was excluded from the angio analysis. The patients who had complete procedural success were only enrolled, and final TFC was repeated after PCI.
Results
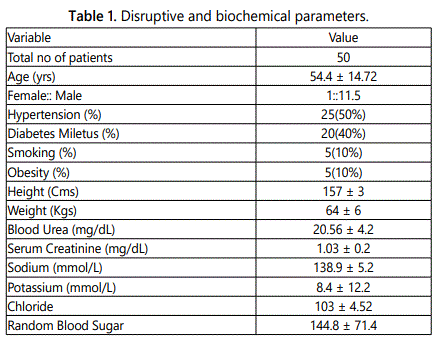
The 50 eligible ACS patients were recruited in this study with the mean age of 54.4 ±14.72 yrs. There were 46 (92%) males and only four females in this study group. Hypertension was in 25 (50%) and Diabetes in 20 (40%) patients. All disruptive and biochemical parameters were mentioned in table 1. The culprit vessel lesion was in LAD in 23 (46%), LCX in 15 (30%) and RCA in 12 (24%) patients (Figure 1).
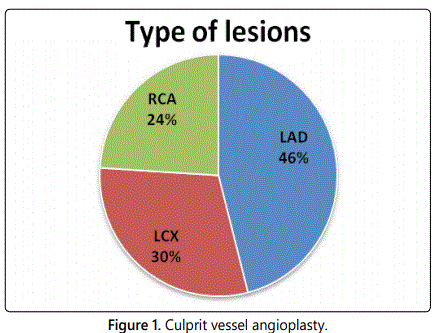
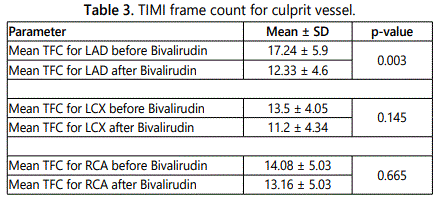
To see the significant change in the TFC we took the mean ± SD of both (before and after) TFC and compared. Mean TFC before Bivalirudin was 16.92 ± 6.2 and after Bivalirudin was 11.4 ± 3.8 which is statistically significant (p=<0.0001) (Table 2, Figure 2). We did sub-analysis for different coronary vessels for the improvement in the TFC. TFC on average for LAD lesions before bivalirudin was 17.24 ± 5.9 and after bivalirudin was 12.33 ± 4.6, which was statistically significant (p=0.003). Mean TFC for LCX, RCA lesions, before and after bivalirudin were not statistically significant. Details mentioned in table 3.


Statistical analysis
The statistical analysis was done with Minitab version 17 (Minitab, Ltd.). The univariate analysis through student two tail t-tests was used for comparison between the two groups. The p-value of ≤0.05 was taken as statistically significant.
Discussion
Bivalirudin is highly specific and binds thrombin at two sites, the active or catalytic site and the fibrinogen recognition site. Slow cleavage of the active-site binding portion of bivalirudin by thrombin itself results in disassociation of the bivalirudin fragments from bivalirudin and exposure of the active site (allowing thrombin to participate in clot formation). Bivalirudin inhibits both circulating and clot-bound thrombin and does not activate platelets. The 25-minute half-life of bivalirudin, together with the mechanism of action whereby thrombin is temporarily disabled but not permanently altered, leads to a superior bleeding profile, with fewer bleeds reported in all trials done with bivalirudin versus heparin in the PCI setting.
Indeed, clinical studies with bivalirudin have shown consistent positive results in PCI patients with stable angina (SA), UA, NSTEMI, and STEMI. Meta-analyses for comparing bivalirudin with unfractionated heparin or enoxaparin plus GPI showed similar mortality rates and ischemic adverse events in patients undergoing PCI but reduced major bleeding complications [5,6].
The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) and the European Ambulance ACS Angiography (EUROMAX) trial showed that bivalirudin primary PCI improved net clinical outcomes after 30 days. There were significant reductions in major bleeding and thrombocytopenia, but an increase in acute stent thrombosis, compared with heparin ± a GPI by a combined data analysis [7].
Matrix [8] and Bright [4] showed an obvious benefit in all-cause mortality where death in the bivalirudin group was significantly lower than in those receiving heparin. In the majority of the studies, the improvement in outcome was demonstrated because bivalirudin decreases the bleeding complications [9]. Other pleiotropic effects of bivalirudin were not mentioned in these trails. We did this study to find out were bivalirudin has any additional benefits than discussed before.
The TFC method has contributed to the understanding of coronary artery disease pathophysiology and has been used to assess the efficacy of intracoronary pharmacotherapy in the setting of acute coronary syndromes. TFC is a simple, cost-effective and widely applicable method and it has contributed significantly since its evolution to the understanding of the physiology and pathophysiology of coronary artery blood flow not only in an infraction but also in many other specific conditions [10].
The use of bivalirudin was associated with greater post-PCI coronary flow reserve compared with the eptifibatide group (1.43 vs. 1.33, p=0.036) as per PROTECT-TIMI 30) trial [11]. Post-procedural coronary flow and enzymatic infarct size compared with the standard intravenous route with Intracoronary bivalirudin bolus administration during primary PCI, is safe and improves ST-segment resolution [12]. PROTECT TIMI trial [11], and Lupi A et al. [12] demonstrated post PCI improvement of coronary flow, but the mechanism was not discussed.
The coronary flow improvement by bivalirudin may be due to additive vasodilatory property or action on the thrombus. Aisha Harun et al. study [13] reveals that bivalirudin also has the same effect of leeching treatment with experience with pharmacologic leeching with bivalirudin for adjunct treatment of venous congestion of head and neck reconstructive flaps. Probably bivalirudin was also showing the vasodilatation as superadded effect along with antithrombotic function. If bivalirudin has vasodilatory property in addition to its anticoagulant effect, probably after PCI, improvement of coronary flow and reserve can be explained. With that hypothesis in the background, we tested where coronary flow improvement occurs even before the PCI. We showed in the present study that TFC before and after bivalirudin bolus followed by infusion showed the reduction in the TFC, reflecting improvement in the coronary blood flow.
Diabetes reduces coronary flow reserve [14]. John A Bittl study [15] demonstrated that the significantly higher incidence of diabetes in the bivalirudin group than in the eptifibatide groups (44.4% vs. 36.4%) did not interfere with the therapeutic advantage of the direct thrombin inhibitor. The primary endpoint of coronary flow reserve still favored bivalirudin treatment [15]. In our study also 40% of the patients had diabetes, but average TFC improvement before and after the bivalirudin was seen which was statistically significant (p=0.0001).
On the basis of the ACUITY trial, bivalirudin monotherapy, in concert with aspirin and clopidogrel, may be considered first-line antithrombin to be started in the emergency department in patients with moderate and high-risk ACS for whom an early invasive strategy is planned [16]. Initial and post-procedural TIMI flows are known to have significant prognostic implication among the patients undergoing primary PCI for STEMI. TIMI flow 3 in STEMI patients before arriving in the cauterization laboratory to improve clinical outcome [17]. For predicting clinical outcomes, CTFC measurements are very useful [18]. So, to improve the TFC, our study also supports to give bivalirudin immediately for the patients who are undergoing for invasive treatment for ACS.
Conclusion
The key finding of our study is that the improvement of TFC with use of bivalirudin during PCI. Bivalirudin seems to improve TFC before angioplasty. Further studies are required to confirm the efficiency of bivalirudin for improvement of coronary flow and to know the mechanism for improvement of coronary flow.
References