Research Article
Detection of Chlamydia trachomatis Antigen in Cervical Secretions and Serum in Infertile Females Undergoing ICSI
1Departments of Obstetrics and Gynecology, Faculty of Medicine, T1anta University, Tanta, Egypt
2Departments of Microbiology, Faculty of Medicine, Tanta University, Tanta, Egypt
*Corresponding author: Mohamed Nabih EL-Gharib, Professor, Department of Obstetrics and Gynecology, Faculty of Medicine, Tanta University, Tanta, Egypt, E-mail: mohgharib2@hotmail.com
Received: January 8, 2019 Accepted: January 21, 2019 Published: January 28, 2019
Citation: Mousa RF, Bohoty SB, El-Shorbagy SH, Hassan AM, EL-Gharib MN. Detection of Chlamydia trachomatis Antigen in Cervical Secretions and Serum in Infertile Females Undergoing ICSI. Madridge J Intern Emerg Med. 2019; 3(1): 105-109. doi: 10.18689/mjiem-1000124
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Chlamydia trachomatis (CT) is the most frequently detected agent of sexually transmitted infections worldwide. It is an obligate intracellular bacteria. CT infection of the lower female genital tract (FGT) can cause cervicitis and if ascending to the upper FGT may result in serious sequelae such as pelvic inflammatory disease, salpingitis and tubal factor infertility.
The purpose of this prospective study is the detection of CT antigen in cervical secretions and serum antibodies in infertile females undergoing intracytoplasmic sperm injection (ICSI).
The study was carried out at Tanta University Hospital in a period from June 2017 to September 2018. It was a prospective observational study, including 40 infertile females undergoing ICSI (patient group), and 20 multiparous women attending the outpatient clinic for contraception (control group).
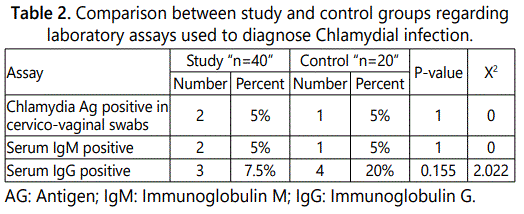
We found that only 7.5% of the study group were positive for serum IgG compared with 20% of the control group. Further, in the study group, we found that serum IgM was found positive only in 5% in both studied groups. Similarly, Chlamydia antigen the cervical swab was detected in 5% of both the study and control group.
The very low prevalence rate of Chlamydia trachomatis infection in Egyptian females is justifiable by the cultural and religious impact on the sexual lifestyle in the Egyptian population. This low prevalence rate of CT infection in Egyptian females minimizes its role as a cause of infertility in the Egyptian population.
Keywords: Gynecology; Inflammatory Disease; Hypertension; Renal Disease; Systemiclupus.
Introduction
Chlamydia trachomatis is the most frequently detected agent of sexually transmitted infections worldwide. It is an obligate intracellular bacteria. They live in an intracellular vacuole that they customize to permit delivery of nutrients and to provide protection against host cell defenses [1].
According to recently published data, global estimates show that at least 100 million are estimated to be infections with C. trachomatis [2]. The prevalence of chlamydial infections was found to be 1.7% in 14–39-year-old women, but 4.7% of sexually active 14–24-year-old women [3]. Therefore, annual screening in females <25 years is strongly recommended [4].
Members of the Chlamydiales are characterized by an extraordinary life cycle, which alternates between two morphological forms, the elementary body (EB) and the reticulate body (RB) [5,6]. The extracellular and metabolically less active EBs are responsible for the dissemination of the bacteria within the infected tissue or to other hosts. Once internalized into a host cell, EBs remain in special vacuoles named inclusions and differentiate to the intracellular, osmotically instable, metabolically more active RBs. These bodies undergo repeated cycles of binary fission before they re-differentiate to EBs, which are released and able to restart the cell cycle [7].
Chlamydial infection of the lower female genital tract (FGT) can cause cervicitis and if ascending to the upper FGT may result in serious sequelae such as pelvic inflammatory disease (PID), salpingitis and tubal factor infertility (TFI) [8].
Several paradigms exist on the pathogenesis of C. trachomatis infections of the urogenital tract and disease progression to infectious infertility [9].
The initial chlamydial infection is typically resolved subsequent to the induction of a robust adaptive immune response, but such immunity only offers partial protection against reinfection [10].
C. trachomatis infects the endocervical epithelia of women and the urethral epithelia in both sexes and is the leading cause of bacterial sexually transmitted infections in humans [11]. Individuals with genital Chlamydia infection often do not exhibit any symptom (75–90% of woman; 30– 50% of men); however, they still can transmit infection [12]. Among the untreated women, 20–40% have involvement of the Fallopian tubes and pelvic inflammatory disease [13]. They also may develop severe sequelae, such as ectopic pregnancy and infertility [14].
Chlamydiae secrete plasmid and chromosome-encoded effector proteins into the host cytoplasm and the inclusion membrane that directly target host proteins and function to exploit host cellular processes [15].
The endocervix is lined by simple columnar epithelium and is the primary site of infection of many STIs, such as Chlamydia trachomatis. The predominant class of antibody in secretions of the FGT is IgG. Very few studies have investigated the role of the local humoral response to C. trachomatis in women [16].
The diagnosis of a C. trachomatis infection requires pathogen detection that can be done using direct immunofluorescence, cell culture, or antigen detection. Cell culture (sensitivity=75%, specificity 100%) is only offered by special laboratories because it requires a lot of time, material and personnel. The first choice in testing methods is an antigen detection, which has a high sensitivity (=90%) and specificity (=98%). Since IgM antibodies only occur in the early phase of the infection; an isolated IgM response without IgG antibodies is not expected [17].
Aim of the Work
The purpose of this prospective study is the detection of Chlamydia trachomatis antigen in cervical secretions and serum antibodies in infertile females undergoing in tracytoplasmic sperm injection.
Patients and Methods
The study was carried out at Tanta University Hospital in a period from June 2017 to September 2018. It was a prospective observational study, including 40 infertile females undergoing ICSI (patient’s group), and 20 multiparous women attending the outpatient clinic for contraception (control group).
Patients were counseled before participation in this study, explanation, and notification of the procedures and its aim was done and a written informed consents were taken from all the patients.
The study protocol was approved by the local research and ethics committee in Tanta University Hospital. There were adequate provisions to maintain the privacy of participants and confidentiality of the data, the patient’s name was replaced by a serial number & her address was confidential.
Inclusion criteria
Exclusion criteria
All cases in this study were subjected to the following: Complete history taking, clinical examination, and investigations including:
Patients underwent controlled ovarian stimulation using the long agonist protocol for pituitary down-regulation. Ovarian stimulation was carried out with:
I. Recombinant follicle stimulating hormone (rFSH) alone.
II. Highly purified human menopausal gonadotropin (HP-HMG) alone. or
III. rFSH combined with HP-HMG.
The initial dose of gonadotropin was individualized for each patient according to age, basal FSH levels, antral follicular count, BMI and previous response to controlled ovarian stimulation. \Dose adjustment was performed according to ovarian response and monitored by means of the vaginal scan [20,21].
The Statistical Package for Social Science (SPSS) was utilized for statistical analysis and tabulation. The following statistical measures were used: Descriptive measures included: count, percentage, minimum, maximum, arithmetic mean and standard deviation. Statistical tests included: Student t test, [22] Chi-square test [23]. The level of significant selected for this study was P less than or equal to 0.05.
Results
This work was carried out on 60 married women dividing into two groups; Study group included 40 females attending fertility clinics for the management of infertility, while a group comprising 20 multiparous females attending the outpatient clinic asking for contraception (a control group). The outcomes of this study are revealed in 6 tables and 2 figures.
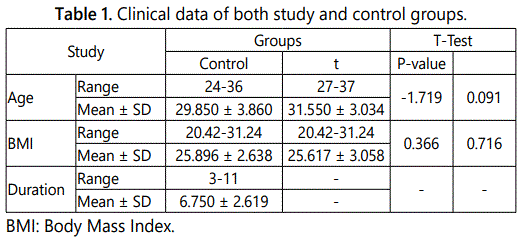
Table 1 displays the clinical data of the examined cases. Where as table 2 provides evidence that there were only 3 positive cases for serum IgG in the study group (7.5%) and 4 positive cases in the control group (20%). Further, in the study group, we found that serum IgM was found positive only in 2 cases (5%), while in the control group it was positive in one case (5%). Chlamydia antigen the cervical swab was detected in 2 cases (5%) in the study group, and in one case in the control group (5%). At that place was no statistical significant difference between the survey and control groups regarding chlamydial antigen positivity.
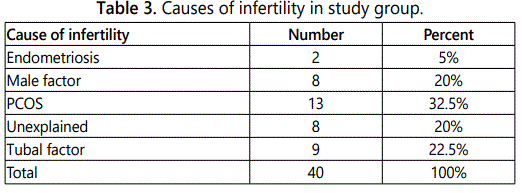
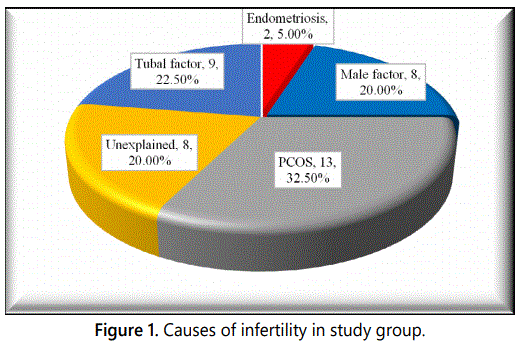
Table 3 and figure 1 demonstrate that the most common cause (32.5%) for infertility was Polycystic Ovaries (PCOS), while the endometriosis was the least (5%). It was also found that there were 8 cases with unexplained cause.
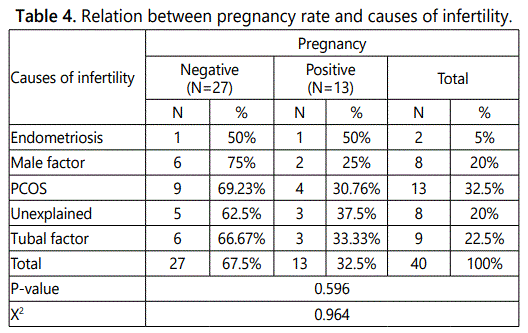
Table 4 shows that by analyzing the pregnancy outcome of the study group cases after ICSI, it was found that there were 13 cases (32.5%) were positive for a pregnancy test (quantitative B-hCG).
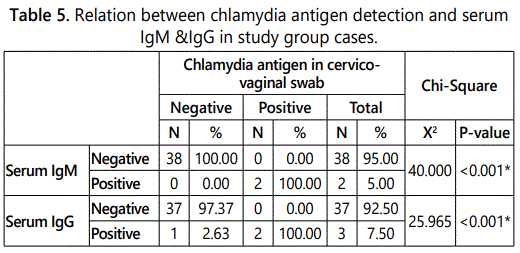
Table 5 illustrates the relation between chlamydial antigen results obtained by cervico-vaginal swabs and serum IgG detection, it was found that in study group there were 38 negative for chlamydia antigen, one case (2.63%) was positive for IgG, whereas in cases positive for chlamydia antigen there were two cases (100%) positive for IgG. It was also found that there were no cases that were positive for IgM in the samples that were negative for chlamydia antigen. In cases positive cases for chlamydia antigen (2 cases) were positive for a positive serum IgM.
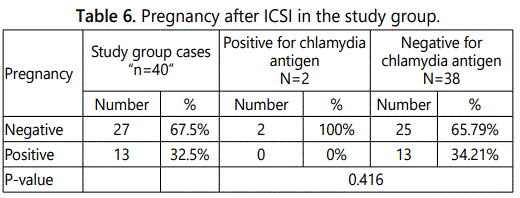
Table 6 shows pregnancy after ICSI in the study group. It was found that 13 cases (34.21%) of cases negative for chlamydia antigen were positive pregnancy test. There were no cases with positive chlamydia antigen got pregnant.
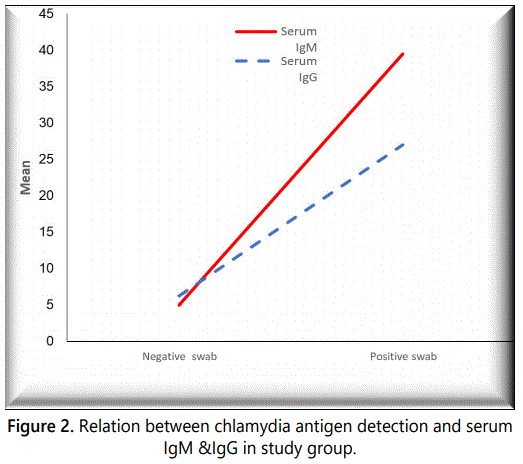
Figure 2 illustrates the relation between chlamydia antigen detection and serum IgM & IgG in the study group.
Discussion
Preexisting asymptomatic infections with Chlamydia trachomatis can be disseminated when infertile patients undergo ICSI, hence, the Royal College of Obstetricians and Gynecologists recommends that all patients undergoing ICSI should be screened for Chlamydia or should have preventive antibiotics [24].
Seropositive women for Chlamydia trachomatis are less likely than seronegative participants to conceive without IVF [25].
In a study on subfertile women with no visible tubal pathology, positive chlamydia antibody testing was associated with a 33% lower spontaneous pregnancy rate than those without chlamydia antibodies [26].
Several methods are used for the diagnosis of Chlamydia infection such as cell culture of cervicovaginal swab, serological testing by detection of specific antibodies: IgM for recent active infection, IgG for previous or chronic infection [27]. Sensing of the nucleic acid of chlamydia organisms via Polymerase chain reaction (PCR) is nevertheless a golden standard for diagnosis, though it is expensive and technically demanding [28].
Antibody testing for anti-Chlamydia antibodies has been found to be associated with tubal factor infertility [29]. Chlamydia IgG antibody test (CAT) is less cost prohibitive than PCR and has less risks than either HSG or laparoscopy [30].
This study was conducted on a randomly selected group of infertile women seeking ICSI, aiming to detect the prevalence of Chlamydia trachomatis infection using ELISA for detection of anti-Chlamydia IgG and IgM and using direct binding monoclonal-based immunochromatographic assay for detection of Chlamydia antigen in cervicovaginal swab.
The results of this study showed no statistically significant difference between age & BMI between study and control groups. This was agreed with Iran et al. [31] & Saleh et al. [32]; who found that there is no substantial difference in age and BMI of the study and control groups.
Regarding causes of infertility in the study group, it was found that the most common reason (23.5%) for infertility was polycystic ovaries (PCOS), while the travelling of the husband and endometriosis were the least (5%). It was also found that there were 8 instances (20%) with unexplained cause.
Miller JH et al. reported that the most usual case of female infertility was male factor (40%) and tubal & pelvic pathology (40%), however, ovarian dysfunction was (15%), unexplained infertility was (10%), unusual problem (5%) (376) Tubal factor infertility due to occlusion and peritoneal pathology causing adhesions is the most usual case of female infertility and diagnosed in approximately 30% to 35% of younger and older infertile women [33].
The lower incidence of positive chlamydia IgG in this field compared to surveys conducted in western rural areas could be assigned to lower frequency of predisposing factors of chlamydia infection including early sexual life and female multiple partners.
Regarding serum IgM, the comparison between the two studied groups showed that there were only 2 cases (5%) in the study group positive for serum chlamydial IgM, while in the control group there was only one case (5%) positive for serum chlamydial IgM, still with no significant difference. A study conducted by Annika Idahl, in Sweden, reported a higher prevalence of positive serum chlamydial IgM, which was about 13% [34].
In the present work, the higher incidence of positive chlamydia IgG in comparison to IgM could be attributed to IgM antibody being, reflecting acute infection while infertile patients have usually chronic infection. Also positive IgG result does not necessarily indicate a genital Chlamydia trachomatis infection, as it could be an infection elsewhere in the physical structure.
The low prevalence of Chlamydia trachomatis (5%) in this study limits the survey of its impact on the success of ICSI cycles. Tasdemir et al. reported no significant impact on the ICSI outcome [35].
On the other hand, Rosenwaks et al. study showed a decrease in embryo characterization in positive cases for chlamydia (Ag), this was justified by heat shock protein induced by an inflammatory reaction in response to infection that may impair embryo implantation and/or facilitates immune rejection after uterine transfer of in vitro fertilized embryos [36].
The very low prevalence rate of Chlamydia trachomatis infection in Egyptian females is justifiable by the cultural and religious impact on the sexual lifestyle in the Egyptian population. Also, Abed-Raboh and associates reported a very low prevalence rate of Chlamydia trachomatis infection in the Egyptian females, which minimizes its role as a cause of infertility in the Egyptian population [37].
Declare any Competing Interests
The authors declare that they have no competing interests.
References