Review Article
Nephrolithiasis; Prevalence, Risk factors and Therapeutic Strategies: A Review
1Department of Eastern Medicine, Government College University Faisalabad-Pakistan
2Department of Pathology, Faculty of Medical and Health Sciences, University of Sargodha-Pakistan
*Corresponding author: Muhammad Akram, Assistant Professor, Department of Eastern Medicine, Government College University Faisalabad, Pakistan, Tel: 345-2888394, E-mail: makram_0451@hotmail.com, makram_0451@yahoo.com
Received: November 20, 2018 Accepted: November 26, 2018 Published: January 3, 2019
Citation: Abbas W, Akram M, Sharif A. Nephrolithiasis; Prevalence, Risk factors and Therapeutic Strategies: A Review. Madridge J Intern Emerg Med. 2019; 3(1): 90-95. doi: 10.18689/mjiem-1000120
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Nephrolithiasis, kidney stone, is a common disease in the World. In industrialized countries, the prevalence of upper urinary tract stones has persistently increased in the twentieth century, yet there are significant contrasts among countries and furthermore inside a similar countries. Most of the people usually can have renal stones at any phase of life. The rate of prevalence of renal calculi is mostly high in males as well as in females. The basic pathophysiology for stone formation is super saturation of components of stones in urine; elements influencing solubility of these components include pH and volume of urine, and total excretion of solute. Majority of the calculi is chemically composed of calcium oxalate. These stones, crystalline in nature and hard, are raised in kidney. The pathogenesis mechanisms of nephrolithiasis are complex and involve both environmental and metabolic risk factors. Increasing prevalence rate suggests that kidney stones are associated with systemic diseases like cardiovascular disease, obesity, and diabetes. Further research of the pathophysiological linkage between kidney stone formation and these systemic disorders is necessary for the development of new therapeutic strategies. Over the past decade, major advancements have been made in the understanding of the pathophysiology, diagnosis, and treatment of renal stones. Treatment strategies for the renal stones are extracorporeal shock wave lithotripsy (ESWL) and conservative medical treatment. Data evidence suggests that therapeutic doses of shock waves may cause acute renal trauma, decrease in physiological functions of kidney, and an increase in recurrence rate of stones. Furthermore, there is no drug that can be satisfactorily used in the treatment of nephrolithiasis. Data collected from In vivo, In vitro, and clinical trials suggest that medicinal plants could be used as an alternative therapeutic strategy in the management of nephrolithiasis. The present review of literature critically evaluates the prospective use of medicinal plants in the treatment of nephrolithiasis.
Keywords: Nephrolithiasis; Calcium-oxalate stones; Extra-corporeal shock wave lithotripsy; Phytotherapeutic agents.
Introduction
Nephrolithiasis is a standout amongst the most widely recognized urinary tract diseases. Current studies estimate the prevalence of renal stones in the United States (US) populace, 7.1% in women, while 10.6% in men [1]. Over the last several decades, the lifelong danger of systemic stones has increased. With these trends, it is necessary for nephrologists, and general practitioner to have recent knowledge of the epidemiology of this disease. Nephrolithiasis, or Renal stones, are common worldwide. Renal stones are the major cause of mortality and morbidity. With its prevalence, increasing continuously, they have a significant economic burden for both developing and urbanized countries. Prevalence of life threatening complication due to nephrolithiasis is 12% [2]. It is suggested that nephrolithiasis can develop in association with systematic disorders such as diabetes mellitus (Type II), dyslipidemia, obesity, and hypertension [3]. Ecological factors along with lifestyle contribute in calculus formation. Renal pain, colic, is a common presentation and consequently the management should not be delayed. Therefore, in the absence of any anticipatory measures, renal calculi recurrence rate is >50% [4]. Pathogenesis of nephrolithiasis, clinical management, prevention and treatment of kidney stones has been summarized in this review.
Prevalence
Symptomatic stones
A number of investigations have stated an increase in incidence rate of kidney stones. An increased prevalence in 20 year to 74-year-old adults was demonstrated by a survey in 1994, from 3.2% in 1976-1980 to 5.2% in 1988-1994. Continuous increase in the overall prevalence to 8.8% was revealed by data analysis of 2007 to 2010 surveys. Incidence history of kidney stones was reported more in males than in women (10.6% vs. 7.1%) [1], with the increase in age group, prevalence of nephrolithiasis was also increased. Variations were also observed in race and civilization [5].
Asymptomatic stones
Asymptomatic stones are comparatively more common. Furthermore, patients are likely to remember only symptomatic stone events.
Incidence
Overall incidence rate had increased, in the United States (US), during 1971 to 1978. In the year 2000, through survey of two large insurance carriers, an incidence of 1116/100,000 was reported for employees (18-64-year-old). A steady increase in incidence from the 1950s through 1990 was estimated by studies performed in Rochester, MN, with a drop somewhat in 2000. In Japan, the incidence of nephrolithiasis has been doubled over a 40-year time period, in both women and men. In the last 10 to 20 years, these increases were most noticeable with rates among men since the 1990s were increasing more sharply, while since the 1980s, the rates increasing more gradually among women [5]. Stone disease has differences in incidence rates between races within the same countries. Followed by Hispanics, blacks, and Asians, incidence rates and prevalence were highest for whites. Within individual races, even from the same race, higher disease risk has been observed in men than in women. Incidence rates for only 1 year was reported by countries and regions include Korea, Seoul, and 4 cities of Spain (Granada, Marina Alta, Tudela, Saragossa).
Types of renal stones
Renal stones formation occurred by presence of more amounts of substances that aggravate stone formation in urine such as calcium, phosphorus, and oxalate. Based on crystalline/ mineral substances in the stone, renal stones are classified into different types.
Calcium stones: In the pathogenesis of calcium-containing renal stones, a significant role is played by dietary intake. These most common types of stones, which occur in two forms: calcium phosphate (15%) and ca-oxalate (75%), of which the calcium oxalates stones are mostly seen [6].
Uric acid stones: Uric acid stones formation has occurred due to high concentration of uric acid in urine. Stone formation is occurred by precipitation of concentrated uric acid in the urine or in conjugation with calcium, with the incidence rate of 8%. Data, from Veterans Administration hospitals, suggested that 12% of stones are composed of some uric acid component and 9.7% are comprised of pure uric acid, in a recent study from Veterans Administration hospitals stone analyses suggested that incidence rate also may varied by the age. In one study, the incidence of uric acid stones in a geriatric population was 11% [7].
Struvite stones: Another type of stone is struvite stone. Major portion in this stone is mineral Struvite with the incidence rate of 1%. Struvite, a magnesium ammonium (MgNH4PO4·6H2O), crystalline substance was discovered in the 18th century for the first time. Struvite urinary stones are also labeled as “triple phosphate stones” and “infection stones” [8].
Cysteine stones: Cysteine stones (<1%) formation is majorly caused by genetic disorders. It causes the leakage of cysteine into kidneys and urine and crystal formation is caused by the disorders that may promote stones growth.
Risk factors
A key role is played by dietary factors to inhibit or promote renal calculus. Other risk factors that aggravate stone formation include genes, fluid intake, environment, and body weight. Risk of renal stones is increased by the following factors:
Dehydration plays key role and a major risk factor for nephrolithiasis; Hereditary incidence of renal stones; high sodium, protein, and sugar diet; Cystinuria; People (commonly females) having infections of urinary tract (UTIs) can suffer mainly of struvite stones; Metabolic disorders (hypercalcemia, hyperoxaluria, low urine volume); Being obese may promotes kidney stones; Hereditary (positive family history); congenital malformations (Medullary sponge kidney, horseshoe kidney, obstructed uretero-pelvic junction); Environmental (hot and arid climate, people working outdoors in warm climate conditions are at risk of stone formation caused by excessive fluid loss) [4]. Vitamin D, oxalate, and various beverages are considered as additional dietary factors that are associated with renal stones. Though the data is restricted, vitamin D supplementation is not accompanied by increased risk of stones, when the goal of supplementation is to overcome vitamin D deficiency in body. Oxalate is identified as a common component of stones. It is found both endogenously as well as exogenously. However, increased oxalate excretion through urine is resulted by increased intake of oxalate rich foods; dietary calcium and supplementation also influence the balance. Therefore, its true link with nephrolithiasis is likely adaptable, and challenging to enumerate. There is association of various beverages with nephrolithiasis. There is a reduced risk of stone formation with increased fluid intake. Coffee and tea also linked with a reduced risk, while there is an associated increased risk of nephrolithiasis due to various sodas. Traditionally, nephrolithiasis was considered a disease that is associated with diet and pathological kidney functioning of electrolytes. Although, it has been suggested by recent investigations that renal stones may be observed as marker of more serious systemic disorders like hypertensive disease, hyperparathyroidism, hyperuricemia, diabetes, obesity, and cardiovascular disease, the self-reported prevalence of renal stones was 61/132 (46%) in patients with increased blood calcium levels and 7/63 (11%) in normocalciuric patients (P<0.0001). Dose-effect association between stone disease and calciuria was found in patients with familial hypercalciuria important. About 40% of patients with renal stones have idiopathic hypercalciuria, which is most probably associated with family history of kidney stones. The risk of kidney stones has increased by high levels of hypercalciuria [9].
Mechanism of Formation of Various Types of Stones
Renal stones are synthesized by salts from the urine that are insoluble in urine and two basic mechanisms have been involved in renal stones formation. The one of the mechanism is characterized by the crystals precipitation with a component, which is non-crystalline protein. Precipitation and then crystallization of the salts occur in the urine. Clinical features are caused by crystals aggregation, and by the crystals that grow into a mass. The other mechanism is mostly responsible for the formation of calcium oxalate stones. In this, deposition of the material components for stone composition mainly occurs on a renal-papillary Ca-phosphate nidus, typically a Randallʼs plaque that always composed by calcium phosphate). The majority of stones are synthesized by mostly of calcium salts, which includes calcium phosphate and calcium oxalate. Remainder of the stones is synthesized by uric acid, magnesium ammonium phosphate (struvite), cysteine stones.
Calcium oxalate stones
Calcium oxalate in unadulterated form or in combination with calcium phosphate is the most common type of urinary stones. Factors leading to stone formation are Idiopathic hypercalciuria, Low urinary citrate, Hyperoxalaluria, Hypercalciuric conditions, and Hyperuricosuria [10].
Calcium phosphate stones (Pure)
Although, mostly the association of calcium phosphate is found with oxalate in most of the calculi, as calcium phosphate stones in pure form are uncommon. Calcium phosphate stones are formed by the high chemical crystallization pressure. Pure calcium phosphate calculi usually are related with renal defects of tubular acidification. In the condition when the normal physiological function of the kidney is lost, necessary to lower the urinary pH, the forms of phosphate (divalent and trivalent) are resulted due to higher level of pH increase, which causes supersaturation of Ca-P [10].
Uric acid stones
On the basis of crystalline composition, uric acid stones are categorized such as, uric acid dihydrate, anhydrous uric acid, sodium, acid urate monohydrate or ammonium acid urate. Thermodynamically, the most stable crystals are of anhydrous form. The dihydrate form (unstable) converts into anhydrous form as it undergoes dehydration. Uric acid dihydrate has been identified in 20% of uric acid stones have been identified as dihydate uric acid crystals. Uric acid stone formation has resulted by the mechanism of uric acid supersaturation. The factors contributing to the stones syntheses are; acidic nature of urine, hyper-uricuria and decreased urine volume. Coexistence of these factors has identified in a specific patient and this leads to nephrolithiasis. Formation of uric acid calculi is mainly promoted by the factors that contribute to the acidic nature of urine. Renal acid excretion partially sustained the normal pH level of urine. Ammonium excretion is the major renal mechanism contributes to correct chronic acid loading is majorly corrected by excretion of ammonium. The pH range of urine 4.8 to 7.4 in normal adults has been reported Urinary pH has been reported to vary, significantly during the day, by diuresis status and diet. A diet with high animal proteins content has been identified to contribute in higher level of acid excretion and lower urinary pH compared than a vegan diet. Urinary pH has been reported abnormally low in the study of idiopathic uric acid stone formers and in a number of patients having gout [7].
Struvite stones
These stones are chemically synthesized by magnesium, phosphate, ammonium along with carbonates. The urinary pH of >7.2 and ammonia in the urine lead to stones formation. Hydroxylation of urea into ammonia and carbon dioxide is catalyzed by Urease. P. mirabilis is the organism, which is most commonly related to these stones. The stone formation has been induced by bacterial infection as the crystal adherence tendency is increased by infection. The lining of bladder mucosa (glycosaminoglycan layer) is damaged by ammonia; in turn bacterial adherence is further increased. Struvite crystals adherence tendency is increased by the damage of bladder lining. Bacterial adherence, production of organic matrix, tissue inflammation, and crystal matrix interaction is facilitated by damage of glycosaminoglycan layer [10].
Cystine stones
Cystine stones are developed by raised level of cysteine in urine. Cystinuria is a genetic disorder with an autosomal recessive trait. Among all naturally occurring amino acids, solubility of cysteine is the least. Cysteine is least soluble at normal pH of urine; therefore, cysteine calculi have been mainly formed in patients with increased cysteine levels of urine [10].
Clinical presentations
The stone passage causes severe pain - is often an initial presentation of nephrolithiasis, which is triggered as the stone move from the pelvis of kidney into the ureter, which leads to the spasm of ureter and obstruct the passage. Pain is originated from flank area, and then referred to the genital areas with the passage of stones downward. With change of position, pain is not usually alleviated, and the pain may be accompanied by vomiting and nausea. Hematuria may be microscopic or gross, but it is always present. Urinary urgency as well as urinary frequency is caused by lodging of stones at the junction of uretro-vesical. All symptoms are relieved quite shortly by the downward movement of stones into the bladder, and flushed out through urination. Hematuria, infection, or loss of renal function is associated with renal pelvic calculi rather than renal pain. Sometimes renal pain is along with microscopic hematuria. Dysuria can also be developed as once the stone has flushed into the lower part of urinary tract. Associated clinical features with renal stones can be mostly categorized into: (i) features of the underlying disorder followed by nephrolithiasis; (ii) clinical manifestations of the stones (iii) clinical features of complications of nephrolithiasis [11].
Diagnosis
Blood test
Blood test is performed to evaluate the uric acid and calcium level in the blood as well as normal renal physiology.
Urine tests
Evaluation of the urine substances is done by this test.
Imaging tests
The location of renal stones in the urinary tract is shown by this test. Imaging tests include X-rays (KUB); CT scan,
ultrasonography (USG), and intravenous pylography (IVP),
retrograde pyelogram, and MRI scan.
Management
There are various treatment options are available depending upon the stoneʼs size and type.
Small stones
These stones typically excreted out on their own from the body without considerable treatment. Intake of sufficient amount of water (04-05 lts a day) may help flush out the stone might be flushed out through urine with sufficient amount of water intake. To relieve the pain caused by stone movement, pain -killers are used. Usually doctors prescribe an alpha-blocker are prescribed by physicians, as relaxation of the ureter muscles are resulted through these medications, also aids to flush out the renal stone more rapidly and with mild pain. Diuretics, drugs that increase the urine flow, may also have chance to flush out the stone are flushed out by the use of diuretics as these drugs reported to increase urine outflow. Stones <5 mm in size will usually flush out there is spontaneous excretion of stones were observed that were less than 5 mm in size, stones >5 mm in size require urologic intervention for removal. For the initial management of stones of diameter <5 mm, some time for stone passage is allowed to watchful waiting in the absence of any structural malformation. NSAIDS or narcotic drugs have been found to relieve pain. Some studies suggest that the use of may accelerate the time to stone passage In appropriately selected patients, α-(1)-adrenoreceptor antagonist, such as tamsulosin, has been reported to accelerate stone passage [12]. Instructions should be given to the patients to strain their urine to collect excreted stones for examination.
Pharmacological treatment: Thiazide diuretics (with sodium restriction) can be used, for ca-oxalate and ca-phosphate stones, to reduce urine calcium. Alkali supplements, such as potassium citrate, can be practiced to increase the concentrations of low levels of citrate in the urine. Urine pH can be raised by using Alkali supplementation, by this mean risk of stone formation potentially increases; therefore, careful monitoring of pH should be done of these patients. Stone passageway has been found facilitated by use of Calcium channel blockers and prednisolone. Long-term restriction of cysteine in dietary intake is not possible and is not efficacious; therefore, to prevent cystine stones, treatment is through medicine penicillamine and tiopronin and with a medication that raises urine pH [4].
Large stones
Excretion of large stones out from the body by their own cannot be facilitated because of large size. Different pathologies such as loss of nephrons, bleeding, or ongoing urinary tract infections (UTIʼs) are caused by these stones. Presence of any signs of urinary tract infection (UTIʼs), failure to take oral fluids, or requires indications of hospitalization and active management are presence of clinical features of renal infection, failure of fluid intake or obstruction of a single functioning kidney.
Extracorporeal shock wave lithotripsy (ESWL): Renal stones are fragmented into small parts by using radiations that produce strong vibratory movements in stones. The small fragments can be flushed out through urination. In the current era, ESWL is treatment of choice, due to its noninvasive nature, requirement of minimal anesthesia and high level of acceptance by patients and physicians. Despite of dominant use of this procedure for management of nephrolithiasis, this procedure is not significant uniformly for all types of renal stones [13].
Surgical treatment
Nephrolithotomy: Nephrolithotomy is generally performed by doctors if large stones inside or adjacent to the kidneys are found. For this procedure, general anesthesia is given to the patient, and then renal stones are removed by means of the telescopic tool. With a high success rate, percutaneous nephrolithotomy is a harmless and operational technique for minimally invasive elimination of stones calculi [14]. Usually,
stones <4 mm in width pass instinctively and greater than 8 mm in width are unlikely to pass without surgical treatment [4].
Laproscopic pyelolithotomy: Laproscopic pyelolithotomy (retroperitoneal) is ideal for hard calculi in pelvis (retrorenal), especially in thin patients. This procedure is used in patients in whom renal calculi fail to respond ESWL and percutaneous procedures [15].
Medicinal plants for nephrolithiasis
Nigella Sativa L: Nigella sativa L. belongs to family Caryophyllaceae, and parts used are seeds [16].
There are a few studies about role of N. sativa and / or thymoquinone in renal stones. Hadjzadeh et al. [17] have reported that oral intake of N. sativa (ethanolic extract) for 30 days not only significantly prevented the formation of calcium oxalate (CaOx) deposits in prevention groups but also decreased CaOx depositions by 57% in treated groups. The extract did not cause any side effect on kidney weight, because for short time the treatment was given. The urine oxalate level also significantly reduced in prevention group compared to other ethylene glycol group and no significant dissimilarity was observed between these two groups. Thymoquinone was also tested, the major component of N. sativa, indicated that intraperitoneal injection of thymoquinone for 28 days prevent and had disruptive effects on the Ca-Oxalate deposits [18,19].
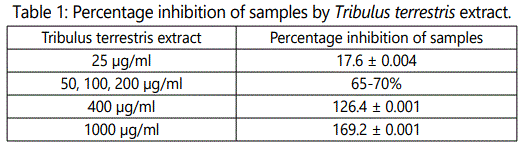
Tribulus terrestris: Tribulus terrestris, belongs to family Zygophyllaceae, is used medicinal plant for renal diseases. Parts used are aerial parts [13]. It is identified to have properties such as pain relieving, diuretic, as well as litholytic properties. Hemostatic properties of this medicinal plant are also reported. It is known for conventional use for the cure of renal stones and also for its diuretic effect. The mechanism of preventing renal stones is that it alters the enzymes associated with hepatic oxalate synthesis as well as glycolate oxidase (GAO) and glycolate dehydrogenase (GAD, hence excretion of urinary oxalate is decreased through this mechanism) [20]. Concentration dependent inhibition manner was shown by extracts of Tribulus terrestris [21] (Table 1).
C. nurvala: The plant C. nurvala belongs to family Capparaceae. Parts used are bark. The effect of C. nurvala bark decoction induced by 3% glycolic acid has been studied in rats on calcium oxalate stones. The decoction showed significant effects in inhibiting the Ca and oxalate deposition in the kidneys by limiting the activity of liver enzymes. Reported effect of bark decoction was identified to lowers the intestinal enzymes levels such as NaZ, KZ-ATPases [22].
Ammi visnaga: The plant Ammi visnaga belongs to family Apiaceae. Fruit decoction of the plant is used. Studies on the effect of seeds on nephrolithiasis have been reported that the stone lysis activity is the major effect of highly potent diuretic, improved hyperbilirubinemia, as well as uraemia [22].
Phyllanthus niruri: It belongs to family Phyllanthaceae. Crystal growth inhibition effect of this plant has been reported [22]. The activity of the extract (aqueous) of this plant on Ca-Oxalate crystal formation process on human urine has also been investigated in in-vitro CaOx precipitation in human urine. Barros et al. [23] observed that the pre-incubation of human urine with Phyllanthus niruri did not inhibit the Ca-Oxalate particles precipitation and even more crystals were obtained in Phyllanthus niruri-containing urine, but the crystals were comparatively smaller than those in urine samples without Phyllanthus niruri [24].
Melia azedarach Linn.: The plant Melia azedarach Linn. belongs to family Meliacea. Renal calculi were induced in male albino wistar rats through ethylene glycol and then effect of aqueous extract of this plant was studied. It shows reduced urinary calcium, phosphate, oxalate levels and elevated urinary magnesium levels as well as urine volume has been reported of aqueous extract of this plant [22].
Asparagus racemosus Wild: Asparagus racemosus Wild belongs to family Liliaceae. The ethanolic extract of Asparagus racemosus Wild had reported to inhibit activity of nephrolithiasis in ethylene glycolated water (0.75%) with induced stones in male Albino Wistar rats having oral administration for 28 days. It has been reported that extract reduced the elevated level of calculogenic ions in urine was significantly reduced and elevations of the urinary magnesium levels was also reported, which has inhibitory effects against crystallization
Plectranthus amboinicus Lour: Plectranthus amboinicus Lour. belongs to family Lamiaceae. The fresh juice of plant Leaves has activity against nephrolithiasis mainly of ca-oxalate origin stones induced by ethylene glycolated water (1%) [22]. Moringa oleifera Lam: It belongs to family Moringaceae. The aqueous and alcoholic extracts of the root wood significant reduction of the elevated levels of urinary oxalate has been reported with the administration of root wood extract of this plant. This study showed a regulatory effect of Moringa oleifera Lam. on the synthesis of endogenous oxalate in ethylene glycol induced hyperoxaluric subject [22].
Cranberry: Urolithiasis risk factors have been reported to affect by cranberry juice significantly. It decreases the excretion of Oxalate and phosphate whereas increases the excretion of citrate. Furthermore, reduced relative super saturation of ca-oxalate was reported [25].
Adiantum capillus veneris Linn: Adiantum capillus veneris Linn belongs to family Adiantaceae. The parts used medicinally are fresh or dried leafy fronds, dried herb with roots and rhizome [26]. Fresh urine sample was taken for 3 hrs. Then, the number of crystals per high-power field was calculated. Hyperoxyluria in Wistar rats was produced. In negative control group A, numbers of Ca-Oxalate crystals were increased. However, hydro alcoholic extract in the dose of 127.6 mg/kg significantly caused the level reduction of Ca-Oxalate minerals. Significant reduction (po0.01) was also reported in test group-B. The data specify that the test drug has significant lithotriptic effect [27].
Herniaria hirsute: Herniaria hirsute belongs to family Caryophyllaceae. In vivo, Cell culture, and In vitro studies was performed. It has diuretic effect and decrease size of crystal [22]. Administration of aqueous extract of Herniata hirsute reduced the crystal deposition in kidneys in calcium-oxalate induced nephrolithiatic rats experimentally [28].
Grapefruit: Grapefruit juice is used for nephrolithiasis. In vivo study was performed on humans. It increases urinary excretion [22].
Aerva lanata: Aerva lanata belogs to family Amaranthaceae. In vivo study on animals was performed. Crystal precipitation was decreased due to medicinal effects of this plant [22].
Administration of aqueous suspension of Aerva lanata (2 g/kg body wt/dose/day) to CaOx urolithic rats, for 28 days, had reduced the level of oxalate-synthesizing enzymes and reduced crystal deposition in the kidney [29].
Pyracantha crenulata: Pyracantha crenulated belongs to family rosaceae. In vivo study on animals was performed. It has diuretic effects and lowers the stone forming constituentʼs concentration [22].
Costus spiralis: Costus spiralis belongs to family Costaceae. Administration of aqueous extract of C. spiralis was found to reduce the growth of renal stones in experimentally urolithiatic induced rats [30].
Conclusion
Stone illness is undeniably basic type of kidney diseases that is linked with mineral deposition in the renal medulla. The review make evident that there has been an expansion in the pervasiveness and incidence frequency of nephrolithiasis. Renal calculi synthesis is due to dietary and ecological elements. Renal stones are most usually seen in the general population regardless of their sex, race, and age. The hazard components can be averted by entirely following suggestions for dietary administration. Treatment of nephrolithiasis is based on eliminating or decreasing supersaturation. At present, in the management of nephrolithiasis, surgical procedures and ESWL are generally used. The main disadvantage of these techniques is that the relapse rate of stones is high. The medicinal plants may without a doubt help in diminishing the repeat rate of renal stones. The present review of literature demonstrates a several of therapeutic plants that are chiefly against Ca-oxalate and Mg-ammonium phosphate kinds of renal calculi.
References
- Ziemba JB, Matlaga BR. Epidemiology and economics of nephrolithiasis. Investig Clin Urol. 2017; 58(5): 299-306. doi: 10.4111/icu.2017.58.5.299
- Hussain M1, Rizvi SA, Askari H, et al. Management of stone disease: 17 years experience of a stone clinic in a developing country. J Pak Med Assoc. 2009; 59(12): 843-6.
- Sakhaee K. Nephrolithiasis as a systemic disorder. Curr Opin Nephrol Hypertens. 2008; 17(3): 304-309. doi: 10.1097/MNH.0b013e3282f8b34d
- Aggarwal R, Srivastava A, Jain SK, Sud R, Singh R, Renal stones: A clinical review. EMJ Urol. 2017; 5(1): 98-103.
- Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010; 12(2-3): e86.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993; 328(12): 833-838. doi: 10.1056/NEJM199303253281203
- Shekarriz B, Stoller ML. Uric acid nephrolithiasis: current concepts and controversies. J Urol. 2002; 168(4): 1307-1314.
- Griffith DP. Struvite stones. Kidney Int. 1978; 13(5): 372-382. doi: 10.1038/ki.1978.55
- Lerolle N, Lantz B, Paillard F, et al. Risk factors for nephrolithiasis in patients with familial idiopathic hypercalciuria. Am J Med. 2002; 113(2): 99-103.
- Balaji KC, Menon M. Mechanism of stone formation. Urol Clin of North America. 1997; 24(1): 1-11. doi: 10.1016/S0094-0143(05)70350-5
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006; 367(9507): 333-344. doi: 10.1016/S0140-6736(06)68071-9
- De Sio M, Autorino R, Di Lorenzo G, et al. Medical expulsive treatment of distal-ureteral stones using tamsulosin: a single-center experience. J Endourol. 2006; 20(1): 12-16. doi: 10.1089/end.2006.20.12
- Lingeman JE, Siegel YI, Steele B, Nyhuis AW, Woods JR. Management of lower pole nephrolithiasis: a critical analysis. J Urol. 1994; 151(3): 663-667. doi: 10.1016/S0022-5347(17)35042-5
- de la Rosette J, Assimos D, Desai M, et al. The clinical research office of the endourological society percutaneous nephrolithotomy global study: indications, complications, and outcomes in 5803 patients. J Endourol. 2011; 25(1): 11-17. doi: 10.1089/end.2010.0424
- Gaur DD, Agarwal DK, Purohit KC, Darshane AS. Retroperitoneal laparoscopic pyelolithotomy. J Urol. 1994; 151(4): 927-929.
- Bahmani M, Baharvand-Ahmadi B, Tajeddini P, Rafieian-Kopaei M, Naghdi N. Identification of medicinal plants for the treatment of kidney and urinary stones. Journal Renal Inj Prev. 2016; 5(3): 129.
- Hadjzadeh MA, Khoei A, Hadjzadeh Z, Parizady M. Ethanolic extract of nigella sativa L seeds on ethylene glycol-induced kidney calculi in rats. Urol J. 2007; 4(2): 86-90.
- Hadjzadeh MA, Mohammadian N, Rahmani Z, Rassouli FB. Effect of thymoquinone on ethylene glycol-induced kidney calculi in rats. Urol J. 2008; 5(3): 149-155.
- Hayatdavoudi P, Khajavi Rad A, Rajaei Z, Hadjzadeh MA. Renal injury, nephrolithiasis and Nigella sativa: A mini review. Avicenna J Phytomed. 2016; 6(1): 1.
- Kieley S, Dwivedi R, Monga M. Ayurvedic medicine and renal calculi. J Endourol. 2008; 22(8): 1613-1616. doi: 10.1089/end.2008.0020
- Aggarwal A, Tandon S, Singla SK, Tandon C. Diminution of oxalate induced renal tubular epithelial cell injury and inhibition of calcium oxalate crystallization in vitro by aqueous extract of Tribulus terrestris. Int braz j Urol. 2010; 36(4): 480-489.
- Yadav RD, Alok S, Jain SK, et al. Herbal Plants Used In The Treatment Of Urolithiasis: A Review. Int J Pharm Sci Res. 2011; 2: 1412-1420.
- Barros ME, Schor N, Boim MA. Effects of an aqueous extract from Phyllantus niruri on calcium oxalate crystallization in vitro. Urol Res. 2003; 30(6): 374-379.
- Boim MA, Heilberg IP, Schor N. Phyllanthus niruri as a promising alternative treatment for nephrolithiasis. Int Braz J Urol. 2010; 36(6): 657-664.
- McHarg T, Rodgers K, Charlton A. Influence of cranberry juice on the urinary risk factors for calcium oxalate kidney stone formation. BJU Int. 2003; 92(7): 765-768. doi: 10.1046/j.1464-410X.2003.04472.x
- Al-Snafi AE. The chemical constituents and pharmacological effects of Adiantum capillus-veneris-A review. Asian J of Pharma Sci and Technol. 2015; 5(2): 106-111.
- Ahmed A, Wadud A, Jahan N, Bilal A, Hajera S. Efficacy of Adiantum capillus veneris Linn in chemically induced urolithiasis in rats. J Ethnopharmacol. 2013; 146(1): 411-416. doi: 10.1016/j.jep.2013.01.011
- Atmani F, Slimani Y, Mimouni M, et al. Effect of aqueous extract from Herniaria hirsuta L. on experimentally nephrolithiasic rats. J of ethnopharmacol. 2004; 95(1): 87-93. doi: 10.1016/j.jep.2004.06.028
- Soundararajan P, Mahesh R, Ramesh T, Begum VH. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006; 44(12): 981-6.
- Araújo Viel T, Diogo Domingos C, da Silva Monteiro AP, Riggio LimaLandman MT, Lapa AJ, Souccar C. Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J Ethnopharmacol. 1999; 66(2): 193-198.


