Review Article
Fetal Hemoglobin Rich Cell Therapy to Combat Adult Anemia
1Head, Department of Regenerative Medicine and Translational Science, Cord Blood Bank, Calcutta School of Tropical Medicine, India
2Research Associate, Department of Regenerative Medicine and Translational Science, Calcutta School of Tropical Medicine, India
*Corresponding author: Niranjan Bhattacharya, Head, Department of Regenerative Medicine and Translational Science, Calcutta School of Tropical Medicine, Kolkata, India, Tel: +91 9830038158, E-mail: sanjuktaniranjan@gmail.com
Priyodarshi Sengupta, Research Associate, Department of Regenerative Medicine and Translational Science, Calcutta School of Tropical Medicine, Kolkata, India, E-mail: priyosengupta85@gmail.com
Received: May 26, 2018 Accepted: June 1, 2018 Published: June 8, 2018
Citation: Bhattacharya N, Sengupta P, Bhattacharya A. Fetal Hemoglobin Rich Cell Therapy to Combat Adult Anemia. Madridge J Intern Emerg Med. 2018; 2(2): 61-66. doi: 10.18689/mjiem-1000113
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Since the first hematopoietic stem cell transplantation in 1989 to treat Fanconi’s anemia, research in cord blood transplantation is currently and predominantly focused on exploiting only the 0.01% of the cord blood component rich in progenitor and stem cells [1]. The rest is often thrown away in the trash or incinerator as biological waste. Since, 1999 the paradigm changed and more and more research is now focused on utilizing the 99.9% of the cord blood and its cellular component. Bhattacharya et al., has reported the first successful HLA randomized, cord blood transfusion in more than 1000 patients suffering from severe anemia at the backdrop of different diseases.
Introduction
Fetal blood or cord blood transfusion is the process by which predominantly nonnucleated fetal blood collected during the time of delivery from the umbilical vein after clamping from the lower uterine caesarean section (LUCS) is administered intravenously after its proper screening for transfusion-related transmitted diseases like HIV-I, II, Hepatitis B, C, Malaria and Syphilis with ABO/Rh blood group matching between the donor and recipient. Due to its superior hematological and oxygen-carrying capacity, cord blood transfusion can be an excellent alternative to adult human and allogeneic blood transfusion [2]. In 1999, Bhattacharya et al., for the first time showed the clinical safety and efficacy of cord blood transfusion in more than 1000 patients with severe anemia in the backdrop of several diseases transfused with HLA randomized, serologically screened and blood group matched [2].
Keywords: Blood transfusion; Tuberculosis; Cancer; Beta Thalassemia; Leprosy; HIV; Diabetes; and Arthritis
In sub-Saharan and poor African nations, where there is a lack of proper access to the basic healthcare facility, screening of adult blood is a major problem along with an acute shortage of properly screened adult blood [3]. Due to high cost in the screening of blood in most of the developing nations, due to high cost, is usually conducted at the macro-cellular level by ELISA method unlike PCR technique in the west [4]. Although ELISA is a reliable method implemented for routine checking of infections in blood, it is not as strong as the PCR method where screening of blood can be done at the molecular level and therefore reducing the risk for the transmission of transfusion-related transmitted diseases which remains in case of certain diseases like Hepatitis C, HIV I & II, as there is an existence of a window period that might not be detected by the ELISA method [4,5]. The blood placental barrier has the distinguished advantage of selectively screening the cord blood during pregnancy thereby reducing the chances of vertical transmission of infections from the mother to the fetus [6].
History of fetal blood transfusion
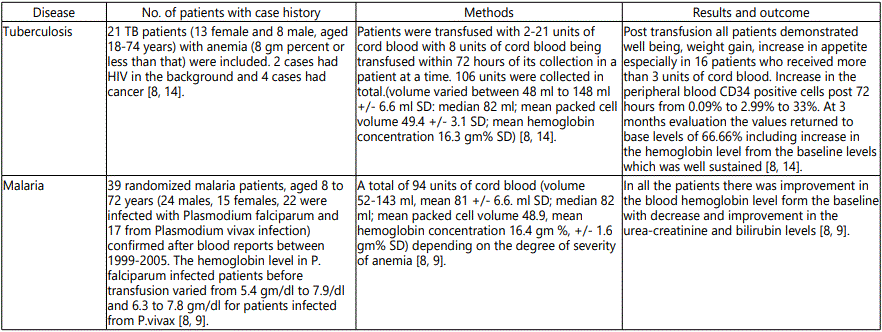
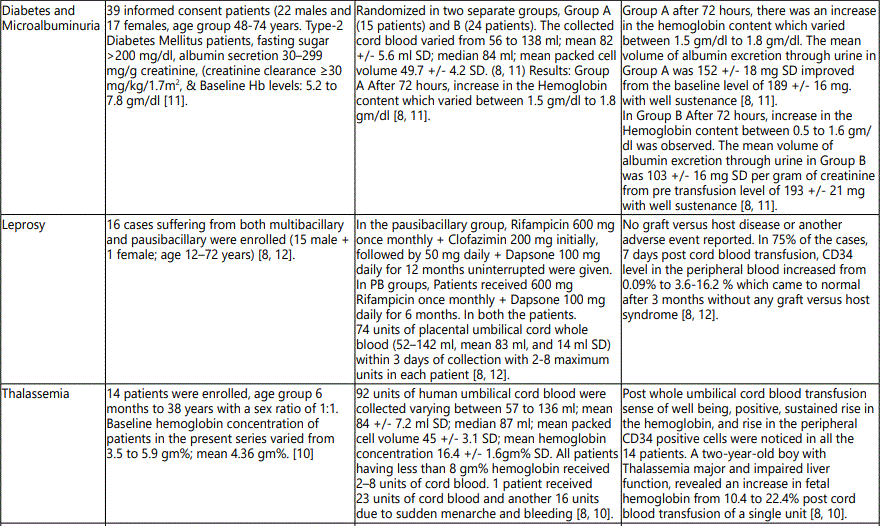
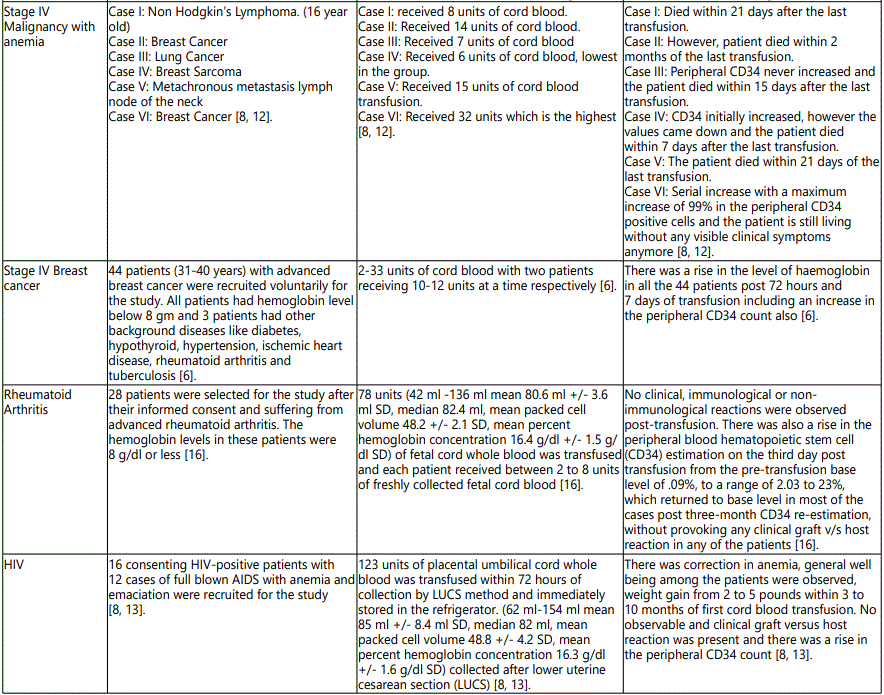
The first attempt to use cord blood as a transfusion substitute was conducted by Halbrecht et al., in 1939 but due to severe limitations in the concept pertaining to the use of anticoagulant, screening of blood and transfusion-related transmitted diseases, the technique could not become a success [4,7]. In 1999, Bhattacharya et al., proved for the first time, the safety and clinical efficacy of cord blood transfusion in more than 1000 anemic patients at the backdrop of several diseases like malaria, tuberculosis, cancer, beta thalassemia, leprosy, HIV, diabetes, and arthritis [8-16].
Bates et al. also reported another study in 2003 showing the clinical efficacy and safety of cord blood transfusion in sub-Saharan children suffering from severe anemia. There was a massive rise in the hemoglobin level in some of the children from the baseline post-transfusion with cord blood [17]. Similarly, in 2003 another study published in the American college of surgeons also highlighted the report of safety and efficacy of 413 units of cord blood transfused to 129 informed consent patients suffering from advanced cancer, systemic lupus erythematosus, ankylosing spondylitis, a plastic anemia, rheumatoid arthritis and thalassemia major with encouraging results without a single case of graft versus and other immune-related reactions [18].
Fetal Blood transfusion: an ideal blood substitute in future
In 2003, wintrobe stated that the ideal substitute for blood transfusion is blood transfusion only. Research into blood transfusion for the last 5 decades has yielded many synthetic and artificial lab substitutes. Normally, a true blood substitute should essentially be able to carry hemoglobin with sufficient oxygen carrying capacity, essential blood components like platelets, cytokines, and immunity against infections [4]. However, there are certain limitations in the new products and techniques developed. Although they can carry oxygen to the tissues, replace platelets and coagulation factors, and bovine based hemoglobin for higher oxygen carry, they are still unable to prove its safety and efficacy on a large scale and most of these methods have failed to cross the FDA Phase III clinical trials [2]. In 2015, Elaine Glickman reported the safe and effective use of fetal cord blood transfusion in India and the possibility of minimizing frequent adult blood transfusion in the future. However, much research is still required before it can be fully established as a clinical practice [19].
Globally every year approximately, 100 million births take place from where roughly 2 to 3 billion liters of properly screened pure fetal blood can be collected for transfusion thereby meeting the global acute shortage of blood transfusion [3]. However, without realizing its true potentiality it is often thrown away in most parts of the world in the trash or incinerated as a biological waste. The properties of human cord blood are unique. The hemoglobin concentration of the fetal blood ranges between 18-22 gm/dl compared to adult hemoglobin where it is between 12 to 17 gm/dl [8]. The cord blood hemoglobin is 70% fetal in nature and has the potentiality to carry 70 % more oxygen than hemoglobin carrying oxygen of the adult blood [8]. The oxygen tension in cord blood is normally 50% saturated and is lower than the adult blood by 6 to 8 mm of Hg pressure. This results in a left shift in the oxygen dissociation curve as 2, 3-diphosphoglycerate has a poor binding capacity with fetal hemoglobin [20,21]. Further, the antigenic expression of the fetal blood is much lower than the adult blood and it is normally 3% to 7%. (22) Cord blood does not express major blood groups and very less expression of Lewis and minor antigens like Kelly and Luther [22]. Also, the platelet count of fetal blood is around 750,000 compared to 150,000 to 450,000 per microlitre of adult human blood. The WBC count is 24,000 per microlitre compared to 4500-10,000 per microlitre in adult blood [8].
Autologus cord blood transfusion in pre-term and low birth infants suffering from anemia and related blood disorders
Pre-term birth neonates with low birth weight, malnutrition, hematological anomalies including surgical interventions post birth immediately suffer from a mild to severe anemia requires a blood transfusion [23,24]. To rectify such cases of neonatal anemia, which is a major and most common complication among neonates in developing countries, application of prophylactic iron substitution therapy, recombinant human erythropoietin, and fetal blood transfusion have helped in reducing the number of blood transfusions required in the initial stages of neonatal life globally [25-29].
Currently, the standard clinical practice for rectifying neonatal anemia is by transfusion of allogeneic blood [23,29]. Majority of the pre-term born low weight babies weighing below 1.5 kilograms requires at least one blood transfusion during the first week of their life [30]. Erythropoietin application in such cases has limited success [25,26]. Immunological, biohazard and parent counseling are some of the important negativities associated with allogeneic blood transfusion [31,32]. Various alternatives are currently being investigated. The application of fetal autologous blood through delayed clamping of the umbilical cord can be an attractive alternative to allogeneic blood transfusion in neonates. Studies have shown that application of fetal blood in premature infants with less than 32 weeks of gestation is associated with marked decrease in the demand for the number of blood transfusions with an increase in the blood volume level when compared to non-fetal blood transfusion during a 3 week observation period, [27,28]. However, problems like hyper viscosity syndrome might be associated with the above method [33].
In 1977, for the first time, autologous cord blood was transfused in an anemic monozygotic twin [34]. In 1979, Paxton et al. reported the application of freshly collected non fractionated, whole autologous cord blood transfusion in 25 asphyxic premature infants [35]. Eichner et al., collected fetal blood from 47 pre-term infants with varying degree of cord blood collection. They could collect only 37 ml of blood from pre-term infants. The group treated 21 infants with 62 units of allogeneic cord blood and 4 were treated with autologous cord blood. They found that 40% of the anemic neonates could be transfused with autologous cord blood transfusions alone [36].
Taguchi et al. also showed in a study that 64% of the infants who had undergone correctional surgery requiring blood transfusion were able to undertake autologous cord blood transfusion without the need for allogeneic fetal blood transfusion [37]. An interesting study by Brand et al., observed that the availability of cord blood transfusion also depended on the gestational week of the infant [38]. Availability of fetal blood was the highest when it was collected from infants born between 20 and 30 weeks of gestation and lowest when it was collected from neonates born between 24 and 28 weeks of gestation [38]. The group also tried to reduce 50% of allogeneic transfusion in infants born less than 32 weeks of gestation using cord blood [38]. They also reported that the infant patient group requiring blood transfusion and born between the 28 to 30 weeks of gestation had the most efficient and productive use of autologous blood transfusion where 42% of the cord blood could be transfused back whereas only 36% of the cord blood collected could be transfused back in infants born between 30 and 32 weeks [38]. However, in infants requiring high transfusion requirement and weighing less than 1000 grams, the process seemed to be ineffective [38,39].
Application of fetal cord blood in acute brain ischemia
In acute ischemia of stroke cord blood transfusion can probably be the best treatment that can be implemented to promote the repair of the ischemic brain post stroke and help in early functional recovery if treatment is provided at the correct time [40]. In ischemic brain injury, reperfusion therapy becomes critical and cord blood due to its high fetal hemoglobin concentration (HbF) can be used effectively as it has greater oxygen binding capacity than adult hemoglobin and can help in neuronal survival in areas requiring high oxygen demand through higher oxygenation [41].
Further evidence from animal studies have also suggested that cord blood therapy in case of acute ischemia stroke and other neurological diseases can promote neovascularization and decrease the level in excitotoxic cellular damage by providing neuro protection against neuro inflammation [42]. Endothelial cells from the cord blood when transplanted into rat diabetic neuropathy models showed increased number of micro vessels [43]. Similar encouraging results like neuro protection against neurotoxicity due to neuro inflammation was observed when human fetal blood was infused into rat heat stroke models [43]. Also, cord blood transfusion can have a positive long lasting effect on relapsing remitting multiple sclerosis due to its neuro-protective role and anti-inflammatory role [44]. Similarly, for motor neuron and neurodegenerative diseases like Parkinson’s disease, placental or fetal blood can have the possibility to provide neuro-protection, decrease the rate of neuro-degeneration with a markedly improved disease free progression.
Conclusion
Cord blood transfusion has immense potentialities to rectify and correct cases of severe anemia and participate in long-term bone marrow reconstitution. One of the significant findings of the above clinical case studies was the rapid rise in the count of the peripheral CD34+ cells post 72 hours of transfusion without any clinical features of graft versus host syndrome and other immunological reactions. This rise in CD34+ cells was independent of any ex-vivo application of growth factors like G-CSF or Valproic acid that might have accounted for peripheral mobilization of CD34+ cells in the host beforehand. The rise could be a direct or indirect effect of the cord blood transfusion. Cord blood is predominantly rich in CD34+ hematopoietic stem cells and one theory might be that the rise in the peripheral count of the CD34+ cells post cord blood transfusion are actually the CD34+ cells of the cord blood transfused. This could seriously challenge the fact that if it so, there will be a graft versus host reaction due to the increase of foreign CD34+ cells in the body which was not evident in any of the clinical cases till date follow up. It might be attributed to the fact that as cord blood is hypo-antigenic in nature, the cellular constitutes of the cord blood are also less antigenic therefore escaping the immune surveillance of the host system through mechanisms which are unknown still. Also, it could be due to the fact also that most of the patients recruited for the study, had a dysfunctional bone marrow resulting in a dysregulated immune surveillance system which failed to recognize the foreign hypo-antigenic CD34+ cells of the cord blood transfused and thereby resulting in no graft versus host action.
Another hypothesis can be that post cord blood transfusion, the cytokines and the growth factors which are present in the cord blood has helped in the endogenous stimulation of the host’s bone marrow which it failed to do so in the absence of cord blood application due to its dysregulation, the causes which are still under intense scrutiny [45]. Cord blood although predominantly rich in hematopoietic stem cells, it has a small fraction of potent mesenchymal stem cells, ectodermal stem cells, very small embryonic-like stem cells (VSEL). Post-transfusion a degree of chimera is expected inside the host’s bone marrow system at the microenvironment level. This certain degree of chimerism then might become essential for stimulation of the host’s bone marrow. However, none of the above theories are confirmed and need more in situ molecular labeling studies in order to understand the fate of the transfused cord blood, its cellular components including that of the host’s endogenous bone marrow system and what causes its stimulation.
In countries, where dengue often takes an epidemic form requiring platelet transfusion, cord blood or platelet-derived from the cord blood can be transfused due to its superior platelet count and in order to manage severe cases of thrombocytopenia. Bhattacharya et al. have further reported, that HLA randomized blood group matched cord blood transfusion need not require any immune-suppression regimen before its application which is very unlikely in cases of cord blood stem cell transplantation where HLA matching is an important step [46]. Also, due to the availability of large quantity of cord blood globally every year, if it is collected, stored and screened properly, it can be also useful in emergency cases such as war, disaster or accidents [47,48].
References