Research Article
Microbial Etiology of Infected Bilomas in Orthotopic Liver Transplant Patients
1Department of Medicine, Division of Infectious Diseases, Rutgers New Jersey Medical School Newark, NJ USA
2Department of Surgery, Division of Transplant and Hepato biliary Surgery, Rutgers New Jersey Medical School Newark, NJ USA
3Penn State Milton S. Hershey Medical Center, Department of Medicine, Division of Infectious Diseases, 500 University Drive, USA
*Corresponding author: Meredith A Schade, Department of Medicine, Division of Infectious Diseases, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033-0850, USA, Tel: 717-531-4633, Fax: 717-531-8881, E-mail: mschade@pennstatehealth.psu.edu
Received: April 11, 2017 Accepted: May 23, 2017 Published: May 29, 2017
Citation: Schade MA, Wilson DJ, Dever LL. Microbial Etiology of Infected Bilomas in Orthotopic Liver Transplant Patients. Madridge J Intern Emerg Med. 2017; 1(1): 3-6. doi: 10.18689/mjiem-1000102
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Bilomas, parahepatic or intrahepatic encapsulated collections of bile, may form following liver transplant in the setting of biliary ischemia and can become infected. Better knowledge of the microbiology of these bilious collections may aid in selecting the most appropriate empiric antimicrobial therapy. We performed a retrospective review of 695 patients who received liver transplants at our institution over a 10-year period and identified 34 with infected bilomas or other biliary complications requiring drainage. Culture results from the patient’s first admission for fever with drainage of a biliary collection following transplantation were reviewed. The majority of infections were polymicrobial and included facultative and aerobic Gram negative bacilli, aerobic Gram positive cocci and a limited number of anaerobes and Candida species. Enterococcus was the most frequent pathogen isolated occurring in 56% of all infections; only 3 patients had vancomyc in-resistant isolates. Six patients had mono microbial infections with multidrug resistant Gram negative enteric bacilli. Our results support the use of broad spectrum antimicrobial therapy for bilomas and biliary collections following liver transplant. The degree of prior antibiotic exposure and the complexity of the liver transplant procedure should be considered when selecting antimicrobial therapy in this population.
Keywords: Biloma Liver transplant; Hepatic artery thrombosis; Biliary is chemia; Enterococcus.
Introduction
The postoperative course following liver transplant is often complicated by infection [1]. Some of the most devastating infections result from ischemia to the biliary tree and associated sequelae. Reconstruction of the biliary tree is performed at the end of the liver transplant procedure, following the hepatic artery anastomosis. Biliary reconstruction has repeatedly been referred to as the Achilles heel of liver transplantation [2]. Hepatic artery stenosis or thrombosis causes ischemic injury to the biliary tree, making the entire allograft vulnerable to infection [3]. Following critical biliary ischemia, bile duct stenosis and necrosis may develop with the potential for bile extra vasation and biloma formation. [4]. Extra luminal collections of bile and biliary strictures are responsible for increased morbidity and mortality, longer lengths of stay and often prolonged intravenous antimicrobial therapy [5]. Limited data is available on the microbiology of infected bilomas and optimal empiric antimicrobial therapy. Our study was performed to gain a better understanding of the organisms involved in the pathogenesis of infected bilomas and biliary tract complications and to improve empiric antibiotic selection in the management of these vulnerable liver transplant patients.
Patients and Methods
University Hospital is an inner city, university-affiliated, tertiary care hospital in Newark, New Jersey. It is the largest liver transplant program in the state of New Jersey and performed an average of 70 adult liver transplants annually during the period studied. A retrospective review of all orthotopic liver transplants (OLT) performed over a 10 year period from January 1, 2002 to December 31, 2012. The study was approved by the Institutional Review Board. Patients were identified through the University Hospital liver transplant database and the Organ Transplant Tracking Record (OTTR). Combined liver and kidney transplants were excluded, leaving 695 cases for analysis. All 695 records were reviewed to determine if infectious complications occurred in the post-operative period. Patients with one or more post-transplant bilomas or biliary strictures were identified by electronic medical record review. Data collected included patient demographics, etiology of liver failure, age at transplant, time to and type of complication, and culture results with antibiograms. Bilomas were defined as intra- or extrahepatic fluid collections on computed tomography (CT), ultrasound or cholangiography, found most commonly in the setting of hepatic artery insufficiency. Hepatic artery thrombosis or stenosis was diagnosed by abdominal ultrasound. Biliary strictures were identified by endoscopic retrograde pancreatography (ERCP) or magnetic resonance cholangio pancreatography (MRCP). Patients found to have bilomas or biliary complications requiring drainage, underwent procedures by interventional radiology to evacuate collections for source control, and to guide antimicrobial therapy. Patients with biliary strictures also had biliary stent placement via ERCP.
Results
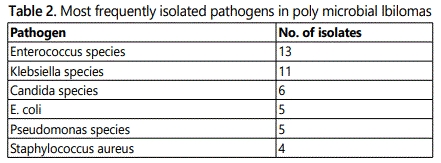
Infected bilomas or biliary tract complications with collections were present in 34 (5%) of our 695 patients following OLT. Table 1 provides a summary of the 34 patients; 25 had bilomas and 9 had other biliary tract complications requiring drainage. Polymicrobial infection was present in 21 patients (62%). The most frequent pathogens recovered are presented in Table 2. Enterococcus was the most frequent pathogen overall, occurring in 56% (19/34) of patients, all of whom had polymicrobial infection. Only three of the 19 patients with Enterococcus had ampicillin and vancomyc in resistant E. faecium (VRE) isolated from aspirated material.
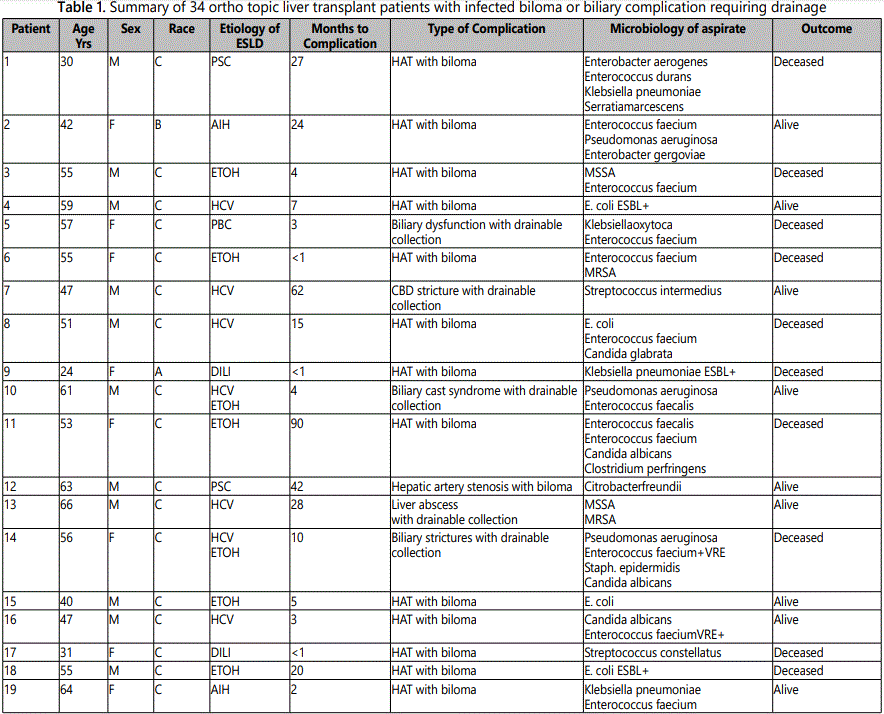
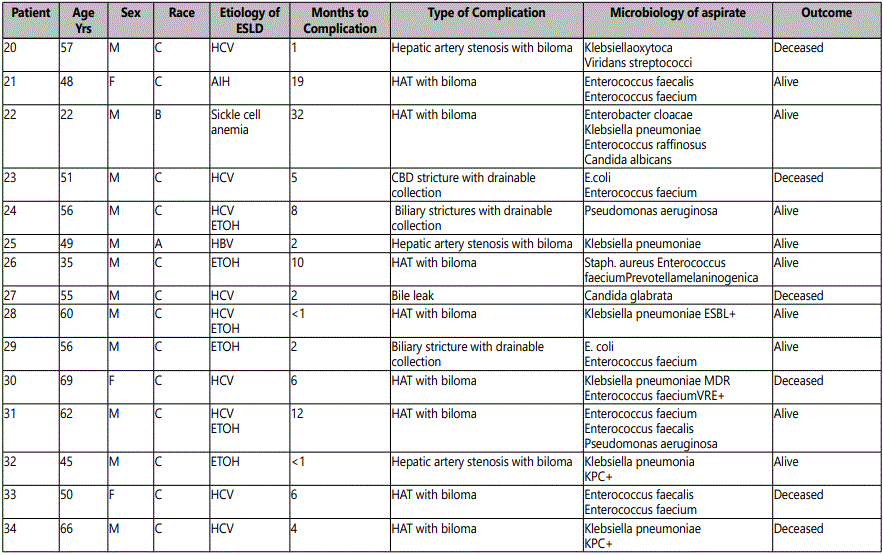
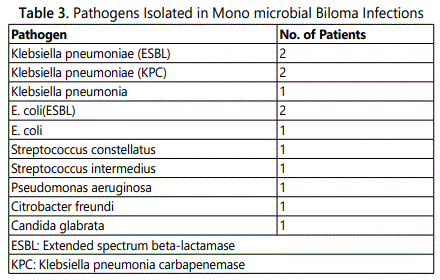
Mono microbial infection was found in the remaining 13 patients as shown in Table 3. Six patients had collections due to a single extended spectrum beta-lactamase producing (ESBL)E. coli or Klebsiella pneumoniae; two of these were carab penemase-producing Klebsiella species. Another two patients in the mono microbial group had infections caused by members of the Streptococcus milleri group, organisms well known to cause serious purulent infection and abscess formation. Only two anaerobes were isolated in the entire cohort, which may reflect the difficulty in recovering anaerobic organisms.
Discussion
Liver transplant recipients are known to be at risk for serious bacterial infections, particularly bacteremia [1,6]. Major factors responsible for infection are the duration and complexity of the transplant procedure, beginning with the native heap tectomy and culminating with the biliary anastomosis. Duration of the average liver transplant is six to 12 hours and is usually associated with transfusion of significant volumes of blood and blood products. Kusne et al. demonstrated a direct relationship between duration of the surgical procedure and number of episodes of infection in a study of 101 liver transplants [1]. The hepatic artery anastomos is can be technically difficult and this artery is the sole supply of oxygenated blood to the biliary tree after transplant. Loss of flow via stenosis or thrombosis of the hepatic artery leads to bile duct ischemia, biliary dysfunction, cholestasis and ultimately rupture, with formation of extra ductal collections of bile known asbilomas [7].
Although bile is normally sterile, stasis of bile due to strictures and biloma formation provides a setting that is conducive to bacterial growth. Bilomas that form post liver transplant may provide a particularly supportive medium for enterococcal growth due to the pathogen’s ability to grow in the presence of bile salts [8]. The presence of necrotic tissue due to hepatic artery insufficiency also compounds the risk of secondary bacterial infection of the bilious collections.
Extensive exposure to broad-spectrum antimicrobials alters the microbial flora of liver transplant candidates [9]. Prior to transplant, patients with advanced liver disease often receive fluoroquinolones for prophylaxis against spontaneous bacterial peritonitis. At the time of transplant, patients receive standard doses of broad spectrum antimicrobials for surgical prophylaxis. At our institution, patients receive intravenous ampicillin-sulbactam pre-operatively, for the duration of the transplant procedure and for 48 hours post-operatively. Ampicillin-sulbactam covers the spectrum of organisms that most commonly cause intra-abdominal infections in the peri-operative period, and most notably has activity against ampicillin-susceptible enterococci. However, it is not active against strains of enterococci that are vancomycin and ampicillin-resistant – most often Enter ococcusfaecium strains. With prolonged antibiotic exposure, there is increased risk for selection of multiply drug resistant organisms [10].
A retrospective review of liver transplant recipients at the University of Illinois at Chicago was conducted to identify patients who developed intra abdominal infection in the first 60 days following transplant [10]. In their cohort of 169 patients there were 104 episodes of infection in 68 patients (40% of the cohort). They identified four types of intra abdominal infection: peritonitis (43%), intra abdominal abscess (29%) enteritis (22%) and biliary tract infection (6%) [9]. Enterococcus was the most commonly isolated organism; 47%of which were vancomycin resistant. Gram negative bacilli were the second most common group of organisms identified and were commonly multidrug resistant.
Bucheli and colleagues prospectively examined the epidemiology of enter ococcal colonization and infection in solid organ transplant patients in the Swiss Transplant Cohort Study, a geographic area with a low prevalence of VRE [11]. They found the highest enter ococcal infection rates in liver transplant recipients, with the majority being E.faecium (38.9%). These infections occurred early in the post-transplant period and were predominantly surgical site infections and intra abdominal infections. An increase in infection-related mortality was not found.
Limited data are available in the literature on the microbiology of collections that form in the setting of biliary tract ischemia following liver transplant. In a study of 492 patients undergoing liver transplant at the University of Wisconsin, a total of 57 patients (11.5%) developed biloma [5]. They found the most common primary infecting pathogen to be enterococcus (37%); half were vancomyc in resistant. Other frequent pathogens in their series included coagulase-negative staphylococci and Candida species. The group noted an association between T tube usage and the presence of coagulase-negative staphylococci. T tubes have not been used at our center in the last decade and only a single coagulase-negative Staphylococcus was isolated in our cohort of patients. Also noteworthy was the limited number of infections due to methicillin-resistant Staphylococcus aureus (MRSA) in our patients. Only two patients (5%) had MRSA recovered from abscess collections.
Our analysis of 695 liver transplants found infected bilomas to be an uncommon complication following liver transplantation occurring in only 5% of our patients. We also found Enterococcus to be the predominant pathogen. Enterococcus is the most common aerobic gram positive coccus in human stool and has previously been considered to be of relatively low virulence [12]. However, in the setting of recent antibiotic pressure, necrotic tissue due to ischemia and the need for immune suppression, the organism appears to take advantage of the post-transplant host. In contrast to the study from University of Wisconsin, vancomyc in resistance was present in a much lower percentage of our enterococcal isolates, all of which were E. faecium.
The AST Handbook of Transplant Infections recommends vancomycin, pipercillin-tazobactam (or a carbapenem) and fluconazole as the primary empiric regimen for patients with possible infected biloma [13]. Our results support the need for broad spectrum antimicrobial coverage that includes an agent active against enterococci, but need not include coverage for MRSA. The prevalence of multidrug resistant pathogens within the health care facility and the community must also be considered when selecting a regimen. Therapy should be appropriately narrowed based on the microbiology of the drained collections.
Author contributions
MA Schade, MD concept and design, data analysis, drafting and critical revision
DJ Wilson, MD concept, critical revision and approval of article
LL Dever, MD concept and design, data analysis, drafting and critical revision
References