Research Article
ANCA Vasculitis in Algeria
1Department of Immunology, Clinique St Therese UHC, Annaba, Algeria
2Department of Nephrology, Hospital IbnSina UHC, Annaba, Algeria
*Corresponding author: Sabiha Gadiri, Professor, Department of Immunology, Clinique St Therese UHC, Annaba, Algeria, E-mail: sabigadiri@gmail.com
Received: January 12, 2019 Accepted: January 29, 2019 Published: February 7, 2019
Citation: Gadiri S, Meriche H, AlliouchKerboua A, Atik A. ANCA Vasculitis in Algeria. Madridge J Immunol. 2019; 3(1): 73-77. doi: 10.18689/mjim-1000117
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis comprises microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic GPA (EGPA). Major target antigens of ANCA associated with vasculitis are myeloperoxidase (MPO) and proteinase 3 (PR3). MPO-ANCA is related to MPA and EGPA, and PR3-ANCA is the marker antibody in GPA.
Objective: We aim to report the clinical-immunological characteristics of 92 patients with positive ANCA vasculitis
Patients and Methods: 92 patients (64 Female et 28 male), with ANCA vasculitis according to the Chapel Hill classification. ANCA was performed by indirect immunofluorescence, supplemented by immune dot to determine their specificity MPO/PR3.
Results: The mean age of patients was 51 years, the diagnosis was: 14 cases of GPA, 21 cases of microscopic polyangiitis (MPA), 04 cases of EGPA, 53 subjects had signs of overlap between the GPA and MPA. The clinical picture was dominated by renal disease followed by lung disease and rheumatologic signs. Some patients had cardiac involvement. 71 patients had p-ANCA (77, 2%), of which 43 was anti-MPO specificity (46.7%), 21 patients had c-ANCA (22.8%), including 9 with a specific anti-PR3 (98%), 40 patients showed no 2 searched specificities (43.4%).
Conclusion: ANCA vasculitis is rare, clinical and immunological spectrum is very heterogeneous. The demonstration of ANCA directed vis-a-vis PR3 and MPO specific as an aid in the diagnosis of systemic vasculitis
Keywords: Vasculitis; Antineutrophil Cytoplasmic Antibodies (ANCA); Myeloperoxidase (MPO); Proteinase 3 (PR3).
Introduction
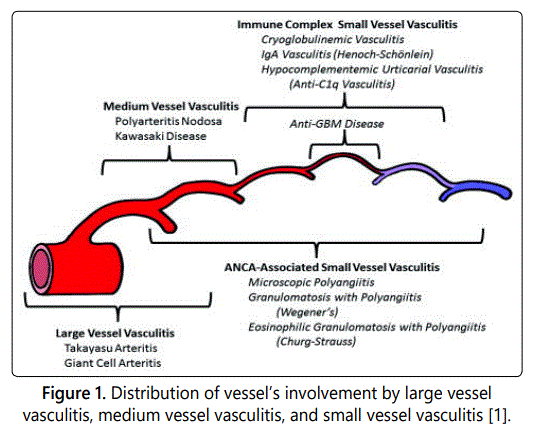
Vasculitis are Vessel's wall inflammation. Vasculitis is classified from the Conference Chapel Hill by size of affected vessels (Figure 1) [1]. ANCA-Associated Vasculitis are affecting vessels of small caliber, they are a collection of relatively rare autoimmune diseases of unknown cause, characterized by inflammatory cell infiltration causing necrosis of blood vessels [2].
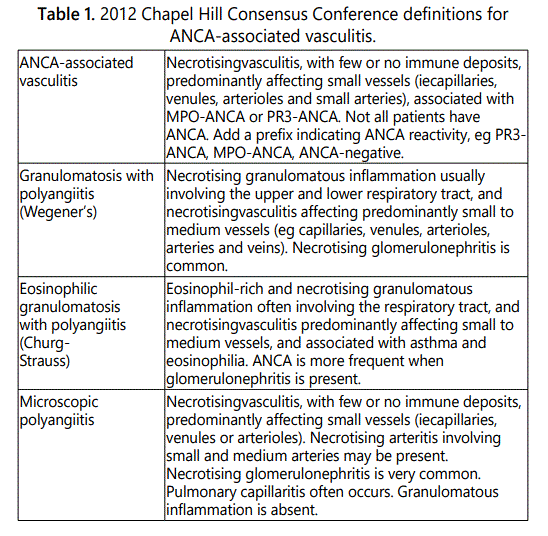
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis comprises microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic (EGPA). Definitions for the AAV were described at the Chapel Hill Consensus Conference (CHCC) in 1994 and were revised in 2012 (Table 1) [1].
Major target antigens of ANCA associated with vasculitis are myeloperoxidase (MPO) and proteinase 3 (PR3). MPOANCA is related to MPA and EGPA, and PR3-ANCA is the marker antibody in GPA.
In c-ANCA reactivity, PR3 is responsible for more than 90% of such reactions. Other antigens may occasionally contribute bactericidal permeability-inducing protein (BPI), rarely MPO.
In p-ANCA reactivity, MPO is responsible for ~10% of such reactivity. Other antigens include lastase, azurocidin, cathepsin Glysozyme, lactoferrin, antinuclear antibodies (ANA) can also have p-anca patterns.
Objective
The objective of this review is to study the clinical and immunological characteristics of 92 patients with positive ANCA vasculitis from the nephrology department.
Patients and Methods
The study was a retrospective study interesting vasculitis patients with patient consent statement, hospitalized in the unit from 2014 October to February 2017. The study includes 92 patients with ANCA vasculitis (64 women and 28 men) according to the Chapel Hill classification. All patients ANCA Positive were included.
ANCA serology was examined by indirect immunofluorescence (IIF) on neutrophil substrate (NOVA Lite ANCA, INOVA Diagnostics Inc, San Diego), regarded as positive, the serums giving a fluorescence >1+. The Titration of the serums (+) takes place on smear of PN fixed at ethanol and, if positive, followed by immunoassays for the detection of antibodies to PR3 and myeloperoxidase MPO (NOVA Lite ANCA, INOVA Diagnostics Inc, San Diego). They are tests ELISA Indirect, which uses micropuits sensitized by PR3 and purified MPO. Upper limits of the normal range were provided by the manufacturer of the assays: MPO 9 IU/ ml and PR3 3.5 IU/ml. Medical records of all patients with one or more positive MPO and/or PR3 ANCA test were reviewed for a clinical diagnosis of AAV (i.e., GPA, MPA, or EGPA). Demographic and clinical parameters were collected: age at presentation, sex, symptoms at presentation, number of affected organ systems, date and level of the first positive ANCA titre, laboratory parameters.
Statistical analysis
Patients of this study were compared with patients with
a clinical diagnosis AAV from other parts of the globe. Chisquare tests were used for categorical data.
Results
Characteristics of patients
During the study period, a total of 92 patients were
hospitalized in the service. The mean age of vasculitis
patients was 51 ± 17 years. Concerning gender, women
represented 69.6% of patients and men represented 30.4%
of patients. Twenty eight were male and sixty four were
females (M:F 1:2). 92 cases satisfied diagnosis of AVV. Of
them 14 (15.2%) had GPA, 21 (22.9%) had MPA, 04 (4.3%)
had EGPA and 53 (57.6%) subjects had signs of overlap
between the GPA and MPA.
Clinical features compared in AAV diseases
The patients present various clinical signs. The majority
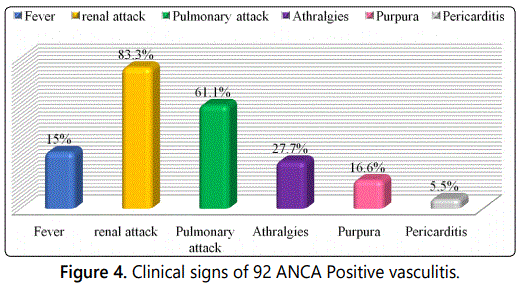
has a renal (83.3%) and/or respiratory attack (61.1%), but
there are also arthralgias (27.7%), purpura (16.6%), fever
(15%) and pericarditis (5.55%) (Figure 4). The renal attack is
the most common clinical sign in patients mainly in the form
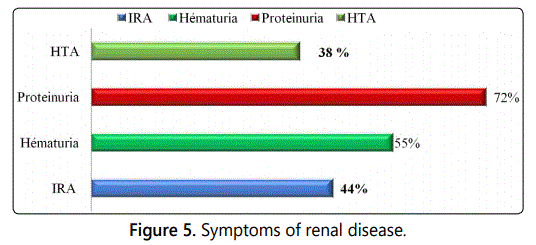
of an IRA (44%) with prevalent proteinuria (72%) and/or
fever (15%). This triad of renal clinical signs is almost
constant, HBP (38%) is less present (Figure 5).
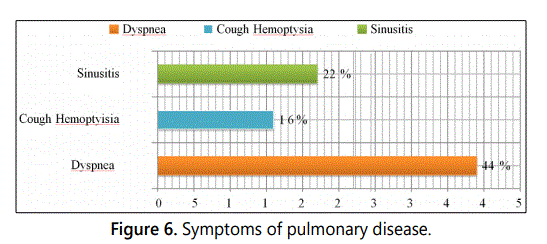
Pulmonary attack is present at a high frequency in patients, represented by dyspnea (44%), cough and/or hemoptysia had at 16%. An ORL attack, in particular sinusian were to find (22%) (Figure 6).
Immunological features of AAV
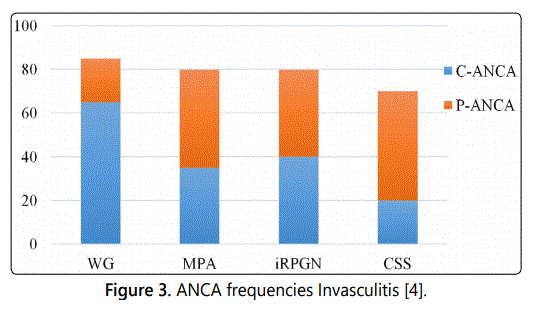
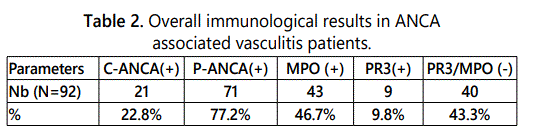
c- ANCA were detected in 21 (22.8%) and p-ANCA in 71
ANCA positive AAV patients (77.2 %) and specificity of ANCA
was PR3 directed in 9 (9.8%) and MPO directed in 43 (46.7%)
of the 71 p-ANCA positive patients tested for PR3 / MPO
specificity. 40 patients (43.4%) showed no 2 searched
specificities (Table 2).
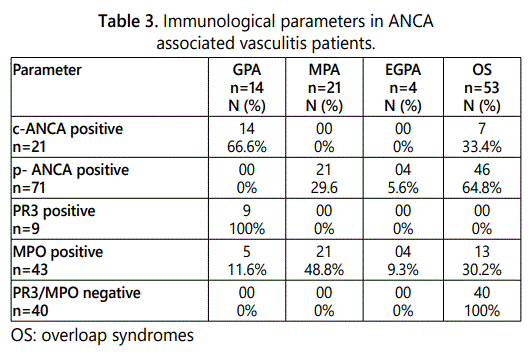
C-ANC As were found in 66.6% of GPAs and 33.4% of overloap (SO) syndromes, whereas P-ANCAs were detected only in 5.6% of EGPAs and 64.8% of S0. Anti-PR3 antibodies were found for 9 cases of GPA, the distribution of anti-MPO antibodies is more heterogeneous, in 5 patients with GPA (11.6%), 21 subjects with an MPA (48.8%) and 13 subjects with a OS (30.2%) and finally 4 patients with EGPA (9.3%) (Table 3).
Discussion
Characteristics of patients
In our study we report our experience with AAV in
Algeria. Many studies have reported geographic variations in
the prevalence of different AAVs. In our series, GPAs and
EGPAs were less frequent than MPAs. The different
frequencies of vasculitis found were significantly lower than
European studies (p<0.05) [2,5]. On the other hand, for
Japanese studies, the frequencies found for GPA and EGPA
were less frequent without significant differences (p<0.1)
whereas for the MPAs in our study the frequencies were
lower than those reported by Sada et al. [6] (p<0.05). As for
the frequency of overlap syndromes (unclassifiable
syndromes), it was more common in our study compared to
Japanese studies Sada et al. [6].
In the literature, AAVs report that men were more frequently affected than women [7,8]. For our study, females were more prominent. The average age of patients in our series was close to those reported in the literature [9,10].
Clinical features compared in AAV diseases
At the time of diagnosis, the general signs (fever) were
reported respectively in 15% of patients and rheumatologic
manifestations in 27.7% of patients. Pulmonary involvement
was observed in 61% with cough and haemoptysis and
dyspnoea. Cutaneous manifestations of the type of vascular
purpura were noted in 16.6% of patients. ENT manifestations
were present in 22% of patients in the form of sinusitis.
Renal involvement was present in 83.3% of patients, revealed
by renal insufficiency in 83.3% of cases, proteinuria in 72% of
subjects and hematuria in 55 % of cases. These clinical signs
have been reported by many authors [11-13].
Immunological features of AAV
ANCA are auto antibodies directed against constituents
of primary granules of neutrophils polynuclear. They are
associated with small vessels vasculitis which includes
granulomatosis with polyangiitis, microscopic polyangiits,
Churg-Strauss syndrome and localised variants of these
diseases (eg. pauci-immune necrotising). As recommended,
ANCA were screened by IFI. When they were positive, their
specificity was determined by ELISA [14,15].
The prevalence of ANCA detected by IFI and the prevalence of MPO and PR3 specificity detected by ELISA were lower than that reported by the Saudi study of A.S. Al Arfaj (p<0.05) [16]. According to Wiik in the generalized and active form of GPA, c-ANCAs are only detected in 73% of cases and ANCA-PR3 between 70%-80% of cases and ANCA-MPO in only 10% of cases [17]. For the GPAs of our series, the frequencies of c-ANCA, and ANCA -PR3 and MPO observed correlate with those reported by Wiik [17-19] and by the Arabic study of AL arfadj [16]. Numerous studies have confirmed the presence of p-ANCA in MPA [20-22]. Micropolyangéitis has generally been associated with ANCAs, more frequently of anti-MPO p-ANCA appearance, rather than anti-PR3 (proteinase 3) [23,24]. The prevalence of anti-MPO p-ANCA ranges from 54% for Ronco et al. [25] to 82% for Gross et al. [26] and Jenette et al. [27]. The frequency of p-ANCAs found in the MPAs of our study was lower than that reported by Tsiveriotis [20] and Savige [15,28] (p<0.05), whereas the frequency of p-ANCA MPO was close to that of Ronco [25]. EGPAs usually associated with ANCAs of p-ANCA appearance with specificity antigenic MPO, are found with a frequency ranging from 40% to 60% [29-31]. Anti-PR3 antibodies of cANCA appearance are present in 10% of cases [32].
The EGPAs of our series had a frequency in p-ANCA MPO lower than those reported by the Arabic and Japanese studies of Al Arfadj and Sada (p<0.05), but the frequency of this antibody was close to the Tunisian study.
In addition, the discovery of high-titre anti-PR3 c-ANCA is predictive of vasculitis more readily affecting the ENT and pulmonary spheres with relapse propensity, whereas observation of high-titre anti-MPO pANCA is more suggestive. vasculitis with renal tropism and reduced relapse potential [33]. For SO, the frequency of ANCA-MPO was lower than that reported by Sada (p<0.05) [6].
Conclusion
ANCA vasculitis is rare, clinical and immunological spectrum is very heterogeneous. The demonstration of ANCA directed vis-a-vis PR3 and MPO specific as an aid in the diagnosis of systemic vasculitis.
References