Research Article
Postprandial Dysmetabolism (Postprandial Hyperglycemia and Hypertriglyceridemia) in Type 2 Diabetes Patients
Chronic Diseases Research Unit, Department of Medical Technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok 65000, Thailand
*Corresponding author: Surapon Tangvarasittichai, Associate Professor, Department of Medical Technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok, 65000, Thailand, Tel: +66-08-9638-8382, Fax: +66-0-5596-6300, E-mail: surapon14t@yahoo.com
Received: April 27, 2018 Accepted: June 11, 2018 Published: June 16, 2018
Citation: Kuntarattanakool M, Isarakul N, Tangvarasittichai O, Tangvarasittichai S. Postprandial Dysmetabolism (Postprandial Hyperglycemia and Hypertriglyceridemia) in Type 2 Diabetes Patients. Madridge J Diabetes. 2018; 2(1): 47-50. doi: 10.18689/mjd-1000109
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Postprandial dysmetabolism [postprandial hyperglycemia (PPG) and postprandial hypertriglyceridemia (PPTG)] was used as acardiovascular disease (CVD) risk factor and as the biomarker for carbohydrates and lipids homeostasis assessment. Postprandial dysmetabolism is an underlying riskin type 2 diabetesmellitus (T2DM) patients even their have good control fasting glucose (FGlu) and triglyceride (FTG) levels. We tried to evaluate the postprandial dysmetabolismin T2DM patients. Twenty fiveT2DM patients, who reached the criteriavolunteered to participate and answered the questionnaires in the present study. Anthropometric and blood pressure (BP) were measured. All patients were received their regular medication. Fasting blood samples were collected for plasma Glu, total cholesterol (TC) and TGanalysis as in Fasting (F) condition.After that all patients received a conventional meal type as their lifestyle in everydaywith a duration time about 10-15 min. Then, blood samples were drawn after 2 hour of this meal load as Postprandialcondition. These patients were older, obesewith good control on FGlu and FTG levels and demonstrated PPG with 41.83% Glu retention and PPTG with 17.30% TG retention after 2 hours of meal load. There is no significant change in FTC and PPTC levels. FGlu, FTC, FTG, PPG and PPTG were significantly correlated with PPG, PPTC, PPTG, % Glu retention and %TG retention. Conclusion, postprandial dysmetabolism is the good biomarker for CVD risk factor and the carbohydrates and lipids homeostasis assessment after meal load, especially in T2DM and obese patient even their apparently good Glu and TG control.
Keywords: Postprandial dysmetabolism; Postprandial hyperglycemia; Postprandial hypertriglyceridemia; Cardiovascular disease risk factor, Type 2 diabetes mellitus.
Introduction
It is well recognized that cardiovascular disease (CVD) is a major complication of patients with and type 1 and type 2 diabetes mellitus (T2DM) [1]. Individuals with diabetes have atwo- to four-fold increased risk of CVD morbidity and mortality [1]. The management of CVD riskremains an important goal of treatment in diabetes patients. Indeed, hyperglycemia, hypertension, obesity, hypertriglyceridemia, and hypercholesterolemia, coexisting conditions that often contributes to CVD in T2DM patients. These comorbidities do not explain all of the excess CVD risk in T2 DM patients.Recent in the understanding of the role of insulin physiology implicate postprandial hyperglycemia (PPG) as the major driver of the increased CVD risk in T2DM patients and prediabetes obesity. Impaired glucose tolerance (IGT) condition was demonstrated as the first-phase of insulin response, while PPG was demonstrated asthe early events in the T2DM progression.
Postprandial dysmetabolism was used as a new biomarker for carbohydrates and lipids homeostasis assessment. The conventional fasting state risk factors were evaluated for CVD risk, while postprandial dysmetabolism was a postprandial state distinguished by abnormally increased glucose and lipids levels in circulation, as the constitutes an independent risk factor for the onset of cardiovascular events [2]. Postprandial hyperglycemia may increase CVD risk by several mechanisms by which insulin may confer as the cardioprotective hormone, as antioxidant, antithrombotic, anti-inflammatory and vasodilatory effects, which are independent of its glucose-lowering activity. Therefore, controlling elevated PPG may a key step to reduce CVD risk in T2DM patients or prediabetes. Postprandial dysmetabolism in lipids is characterized by elevated triglycerides (TG) levels and its remnant lipoprotein particles (RLPs), as postprandial hypertriglyceridemia (PPTG) [2]. Insulin also plays a major role in the regulation of lipid homeostasis and balancing between lipolysis and lipogenesis, since it stimulates lipid synthesis and adipogenesis and inhibits lipolysis [3]. This present study tried to evaluate the postprandial dysmetabolism occurred in T2DM patients as CVD risk.
Materials and Methods
Subjects
A total of 25 T2DM patients (overt diabetes >10 years) were
randomized from the general population in our district. These
T2DM patients were diagnosed and received medications from
any hospital in our district during Novemberto December 2017.
All T2DM patients were given regular treatment with glycemic
lowering, lipid lowering, and anti-hypertensive medication.
Exclusion criteria wasun-control glucose and TG levels, heart
failure, peripheral vascular disease, recent myocardial infarction,
unstable angina, stroke, acute or chronic infection, cancer,
hepatic disease, acute illness. All participants gave written
informed consent. Our study protocol was approved by the Ethic
committees of Naresuan University.
Physical and Biochemical Examination
All T2DM underwent anthropometry, blood pressure
measurement and physical examination. Body mass index
(BMI) was calculated andwaist circumference (WC) was
measured at the midpoint between the rib cage and the top
of lateral border of iliac crest at minimum respiration. BP was
measured after the participants had been seated and rested
for 5 minutes, as the mean value of at least two measurements
for these participants on the same day with calibrated desktop
sphygmomanometers. Venous blood samples were collected
from all participants without stasis after 8-12 hour fast in a
seated position as Fasting (F) condition.
Oral Glucose Tolerance Test (OGTT)
A 75-g of glucose was performed following after the
overnight fasting blood drawing as in the conventional
method. But in present study, all T2DM patients received a
conventional meal type as their lifestyle in every day with
aduration time about 10-15 min. Then, blood samples were
drawn at 2 hour after meal load as postprandial condition.
Percentage Retention of Glucose and Triglycerides
The formula: [%Matter retention = (Concentration of
matter at Postprandial condition - Concentration matter of at
fasting condition)* 100/ Concentration matter of at Fasting
condition] was used to estimate percentage retention of Glu
and TG in plasma at the end of 2 hours. Blood specimens were
processed and assayed in the clinical laboratory of Chronic
Diseases Research Unit, Department of Medical Technology,
Faculty of Allied Health Sciences, Naresuan University,
Phitsanulok, Thailand. Plasma glucose (Glu), serum total
cholesterol (TC) and triglycerides (TG) were measured by
enzymatic method by using calibrators and control materials
from Roche Diagnostics (Roche Diagnostic, Switzerland).
Classification of Glucose and Triglycerides Tolerance
Postprandial glucose tolerance was classified according to
WHO criteria [4] as follows: (i). Normal glucose tolerance (NGT):
Post load glucose <140 mg/dl (ii). Impaired glucose tolerance
(IGT): Post load glucose 140-200 mg/dl (iii). Diabetes: postload
glucose ≥200 mg/dl. According to the National Cholesterol
Education Program [5], patients can be divided into 4 groups
according to their TG levels: (i). normal TG (< 150 mg/dL), (ii).
Borderline-high TG (≥ 150-199 mg/dL), (iii). high TG (≥ 200 - 499
mg/dL), and finally, (iv). very high TG (≥ 500 mg/dL).
Statistical Analysis
All data are presented as median and interquartile range
for non-normally distributed data, tested by using Shapiro-Wilk test. All clinical characteristics of Fasting condition and
postprandial condition were compared by using Wilcoxon
Signed Ranks test and bivariate correlation between these
clinical variables was assessed by using Spearman rank
correlation test. All tests were two tailed, and p-values less
than 0.05 were regarded as statistically significant. All analysis
was performed by SPSS version 13.0 (SPSS, Chicago, IL, USA).
Results
These T2DM patients were older, had a longer duration of diabetes [15.4 (9.1-18.7)], obesity and abdominal obesity with good control oftheirblood pressure, glycemic and lipidemic status at Fasting condition. While demonstrated postprandial dysmetabolism, [(PPG: 12 were mild PPG and 13 severe PPG) and [(PPTG: 6 were normal PPTG, 10 wereborderline-high TG and 9 were high TG) as shown in Table 1.
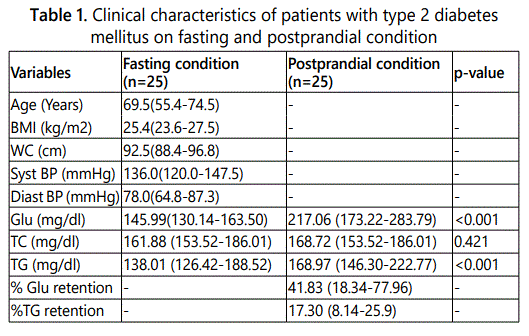
The results demonstrated postprandial dysmetabolism with 41.83% (18.34%-77.96%) of Glu retention and 17.30% (8.14%-25.9%) of TG retentionin circulation after 2 hours of meal load. There is no significant change in FTC and PPTC levels. Bivarate correlation, FGlu, FTC and FTG were significantly correlated with each other of PPG, PPTC and PPTG (r=0.583, p=0.002; r=0.789, p<0.001, and r=0.711, p<0.001) respectively, while PPG, PPTGwere significantly correlated with %Glu retention and %TG retention (r=0.610, p<0.001; r=0.549, p=0.004) respectively, as shown in Table 2.
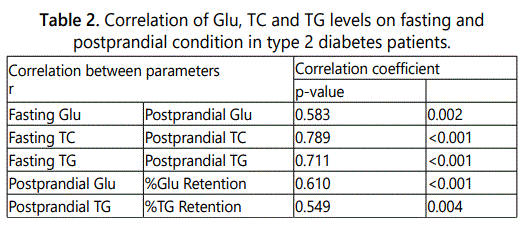
These correlations may result from the linear insulin action or dysregulation in their physiological conditions, with the same insulin sensitivity in each phase of action.
Discussion
As expected, blood Glu levels were higher in T2DM patients at fasting state and remained higher at the postprandial state. Because of the plasma insulin response to the meal was more important in T2DM patients. Insulin action on Glu and TG homeostasis is to reduce the PPG and PPTG levels as follows: (i). Increased glucose uptake by peripheral tissues (skeletal muscles) is mediated via glucose transporters proteins, GLUT4, a major regulator of whole-body glucose homeostasis and stimulated by insulin [6]. (ii). promoted glycogen synthesis (iii) inhibited glucagon secretion, inhibited or reduced Glu and VLDL production by the liver [7]. Hence, the postprandial dysmetabolism is determined by carbohydrate and lipid absorption, insulin dysregulationand glucagon secretion, and their coordinated effects on Glu and TG metabolism in the liver and peripheral tissues.
Postprandial hypertriglyceridemia is more frequent in patients with T2DM and insulin resistance states. In the present study, many of these T2DM patients, despite normal FTG levels, had TG levels >200 mg/dl during the postprandial condition. This condition may indicate that most T2DM patients have plasma TG above the desired level for many hours after the meals. Moreover, the present study suggests that TG levels at fasting state may not always a good predictor of the normal TG metabolism in the postprandial state.In theseT2DM patients, there are multiple abnormalities of lipoproteins of both endogenous and exogenous origin.
There are a large number of lipoprotein particles, as TG levelsincreased in these T2DM patients. This rising was used as a marker of the postprandial dysmetabolism state. Therefore, T2DM patients have PPG and PPTGeven ifthey had good control for their FGlu and FTG normal levels.
Many research studies demonstrated that PPG and PPTG increase oxidative stress by elevated ROS production, as the major role of the diabetes complications, such as activated prothrombotic pathways [8], increases intercellular adhesion molecule-1 (ICAM-1), proinflammatory cytokines and transcription factors, such as nuclear factor kappa B (NF-κB), activator protein-1, early growth response factor-1 (Egr-1), hypoxia-inducible factor-α [9], increased carotid intimamedia thickness [10], and endothelial dysfunction and atherosclerosis [11]. Thus, these T2DM patients with postprandial dysmetabolism (PPG and PPTG) are at CVD risk. Hence, it is important to identify postprandial dysmetabolismof T2DM or any insulin resistance state patients who apparently have good metabolic control, but may at CVD risk. Plasma Glu and TG levels of PPG or post meal load test are more powerful predictor of CVD risk and progression of diabetic retinopathy than HbA1C or FPG levels [12, 13]. While PPTG was demonstrated more potent predictor of CVD risk than fasting TG [14].
Guidelines for management and treatment of PPG in T2DM recommend to use self-monitoring of blood glucose (SMBG) for glucose levels assessment, but may design the timing and frequency of SMBG for individualized with T2DM [15]. Fortreatment of postprandial dysmetabolismare dietary modification, exercise, weight loss and medication (insulin analogue, meglitinides (repaglinide and nateglinide), GLP1 receptor agonists (exenatide, exenatide LAR, liraglutide, lixisenatide), DPP4 inhibitors (sitagliptin, vildagliptin, saxagliptin, alogliptin, linagliptin), statins, ezetimibe, fibrates). Insulin degludec is developed as long-acting insulin analogue, insulin as part is fast acting insulin analogue which allows patients to adjust their insulin according to any changes [16, 17].
Conclusion
Postprandial dysmetabolism as PPG and PPTG is a nonfasting state demonstrated by elevated levels of plasma glucose and triglyceride after meal load. Many research and epidemiology studies demonstrated that postprandial dysmetabolism contributes to developatherosclerosis and increased CVD risk morbidity and mortality. Postprandial dysmetabolism in T2DM patients contributes to cause macro and micro vascular complications from the development of endothelial dysfunction and cardiovascular damage via oxidative stress and inflammation activation.
Acknowledgement
We sincerely thank Naresuan University and all co-workers of Clinical Laboratory of Department of Medical Technology, Faculty Allied Health Sciencesfor their technical assistanceand supporting. We especially thank those who participated and donated blood samples for this study. Finally, we sincerely thank Asst. Prof. Dr. Ronald A. Markwardt, Burapha University, for his reading and correcting of the manuscript.
Conflict of interest
The authors declare that they have no competing interests.
References