Research Article
Ankle Brachial Index (ABI) measurement associated with High Sensitivity-C-Reactive Protein, Insulin Resistance and Pulse Pressure Levels in Type 2 Diabetes Mellitus Patients
1Division of Echocardiogram, Buddhachinaraj Hospital, Phitsanulok 65000 Thailand
2Department of Cadio-Thoracic Technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok, 65000 Thailand
3Chronic disease research Unit, Department of Medical Technology, Faculty of Allied Health Sciences, Naresuan University,
Phitsanulok, 65000 Thailand
*Corresponding author: Surapon Tangvarasittichai, Associate Professor, Department of Medical Technology, Faculty of Allied Health Sciences, Naresuan University, Phitsanulok, 65000, Thailand, Tel: +66-08-9638-8382, Fax: +66-0-5596-6300, E-mail: surapon14t@yahoo.com
Received: February 6, 2018 Accepted: February 22, 2018 Published: March 1, 2018
Citation: Kanokphichayakrai K, Kaewmahanin W, Tangvarasittichai O, Tangvarasittichai S. Ankle Brachial Index (ABI) measurement associated with High Sensitivity-C-Reactive Protein, Insulin Resistance and Pulse Pressure Levels in Type 2 Diabetes Mellitus Patients. Madridge J Diabetes. 2018; 2(1): 31-35. doi: 10.18689/mjd-1000106
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
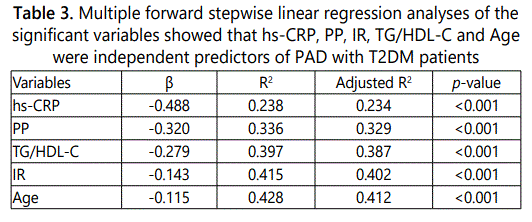
Atherosclerosis is common occurrence in type 2 diabetes mellitus (T2DM) patients. Peripheral arterial disease (PAD) is a major arteries disease caused by atherosclerosis as a vascular complication of T2DM. It can be detected by using ankle brachial index (ABI) measurement. A total of 187 subjects were recruited in present study and underwent ABI measurement. Thirty one of T2DM patients were abnormal low ABI≤0.9 (as Gr-1) and 156 non-T2DM subjects were normal ABI>0.90 (as Gr-2). Comparison of clinical characteristics of these two groups, Gr-1 were significantly increased in pulse pressure (PP), dyslipidemia, insulin, insulin resistance (IR) and high sensitivity-C-reactive protein (hs-CRP) (p<0.05) than Gr-2. Multiple forward stepwise linear regression analyses of the significant variables showed that in these decreased ABI, independent predictors of decreased ABI were hs-CRP (β =-0.488, R2 = 0.238, p<0.001), PP (β = -0.320, R2 = 0.336, p<0.001), triglyceride/high density lipoprotein-cholesterol (TG/HDL-C) ratio (β =-0.279, R2 = 0.397, p<0.001), IR (β =-0.143, R2 = 0.415, p<0.001), and Age (β = -0.115, R2 = 0.428, p<0.001). In conclusion, abnormal ABI≤0.9 or PAD is associated with increased PP, inflammation, IR, dyslipidemia and age. ABI measurement is a useful tool to estimate PAD and cardiovascular diseases risk marker in asymptomatic patients.
Keywords: Ankle brachial index; Peripheral arterial disease; Atherosclerosis; Highsensitivity C-reactive protein; Pulse pressure; Insulin resistance.
Introduction
Atherosclerosis progression is the process affected by the alteration of vascular endothelial cell beds with clinically demonstrated life threatening consequences including coronary artery disease (CAD), cerebrovascular disease, and peripheral arterial disease (PAD) [1]. Peripheral arterial disease is one of the common evidence of atherosclerosis. The prevalence of PAD was increased with age, hypertension, diabetes and smoking [2-5]. People with PAD often have lower extremity circulation problems and they are at higher risk for both cardiovascular disease (CVD) and cerebrovascular disease event [2]. Peripheral arterial disease is commonly assessed by using ankle brachial systolic blood pressure index (ABI) measurement [6, 7]. The ABI result is the ratio of Doppler- or sphygmomanometry-determined lower extremity blood pressure to brachial artery blood pressure. Reproducibility of the ABI measurement is good with the mean error of 8-9% within or between measurements [8]. Peripheral arterial disease severity is assessed according to the levels of ABI: (i) 0.91-1.30: normal, (ii) 0.70-0.90: mild occlusion, (iii) 0.40-0.69: moderate occlusion, (iv) <0.40: severe occlusion and (v) >1.30: poorly compressible vessels. The American Diabetes Association recommends measuring ABI in all adults older than 50 years and history of smoking, hypertension or diabetes or in any patient having PAD and other CV risk factors [9].
C-reactive protein (CRP) is an inflammation marker recommended for risk assessment for primary prevention of cardiovascular disease [10, 11]. Increased CRP level is associated with increased risk of cardiovascular disease [12] and increased vascular stiffness can be caused by atherosclerosis and aging.
Age related increases in blood pressure (BP) usually show the systolic blood pressure (Syst) elevation while maintaining or having a slight decrease in a diastolic BP. This induces in the widening of pulse pressure (difference of systolic and diastolic blood pressure) [13]. However, BP can be divided into two components: (i) steady (mean arterial pressure; MAP) and (ii) pulsatile (pulse arterial pressure, PP) [14]. Previous research has linked elevated PP (estimate of arterial stiffness) with a higher risk of cardiovascular morbidity and mortality [15, 16]. Arterial hypertension (steeper age-related widening of PP) also promotes vascular stiffness [17]. High peripheral resistance is the hallmark of arterial hypertension, but always exerts hemodynamic changes that could counteract the effect of the increase in MAP on PP. In this regard, peripheral resistance maintains a reciprocal relationship with stroke volume and PP [18]. In the present study, we aim to demonstrate that PAD (ABI<0.9) is associated with increased PP, inflammation, IR, dyslipidemia and age. ABI measurement can use as the tested tool to estimate subclinical atherosclerosis and CVD risk in patients with PAD.
Methods
Study Population
Two hundred and five of female participants from the
Cardiovascular Diseases in Diabetes Patients Project during
October 2012-December 2013 were used in the present
study. Volunteers were excluded, if they had an ongoing
febrile illness, history of a connective tissue disorders, non-atherosclerotic arterial disease, history of lower extremity
bypass or percutaneous angioplasty in the preceding year
and those with an ABI >1.3 caused by poorly compressible
arteries in the lower extremities. Eighteen participants didn't
participate in ABI measurement section was excluded from
the study. The number of eligible participants was 187 in the
present study. Thirty one subjects with T2DM were identified
as PAD with abnormal low ABI ≤0.9(Gr-1) and 156 subjects
without T2DM were identified as without PAD with normal
ABI &gre;0.9(Gr-2). The research protocol was approved by the
Ethics Committee of the Naresuan University. All participants
gave informed consent before their provided blood samples
and underwent assessment for ABI measurement.
Anthropometric, Blood Pressure and ABI Measurements
Questionnaires were used to record clinical characteristics
including diagnosis of hypertension, diabetes, a history of MI
or stroke, smoking, alcohol use, and medications of each
participant at the study visit. Anthropometric measurements
of the study included height; weight and waist circumference
(WC).The body mass index (BMI) was calculated from height
and weight as kg/m2. Blood pressure (BP) was measured by
using Omron HEM-7080 (Omron Health care, Tokyo, Japan).
Pulse pressure (PP) was determined by subtracting the diastolic
from the systolic blood pressure (Syst), and mean arterial
pressure (MAP) was calculated by using the formula: [(systolic
blood pressure) + (2 x diastolic blood pressure)]/3 [19].
Ankle brachial index measurement is made in the supine position after 5 min of rest by using Sphygmanometer and Sphygmograph (Vasera, VS-1500N ver. 04; Fukuda Denshi, Japan). A pneumatic-cuff is placed around the ankle and the pressure is measured at both the dorsalis pedis and posterior tibial arteries using a hand held continuous wave Doppler probe (5-10 MHz). We used the same technique measurement in both arms for brachial artery pressure. The higher of the two ankle pressures is divided by the brachial artery pressure. In subjects with normal lower limb arterial circulation, the systolic pressure at the ankle is usually 10-15 mmHg higher than the arm measurement, it caused from pulse wave velocity [20], resulting in an ABI >1.10. Following the recommendation of the International medical societies for the ABI calculation, the highest pressure in the leg is divided by the highest pressure in the arm [9, 21-22]. Reproducibility of the ABI measurement seemed to be good. In the ABI study, the mean error of 8-9% within or between observers is smaller than with established screening measures [8]. The lower of the resting ABI values for the right and left arms and legs was used in the analyses involving the ABI. PAD was defined as an ABI ≤ 0.9 in both arms and legs.
Blood Sample Collection and Biochemical Determination
Fasting venous blood was collected from all participants.
Plasma glucose (Glu), blood urea nitrogen (BUN), total
cholesterol (TC), triglycerides (TG) and high density lipoprotein
cholesterol (HDL-C) were measured by enzymatic method
(Roche diagnostic, Switzerland). Serum creatinine (CT) level
was determined based on the Jaffe reaction. LDL-C level was
calculated with Friedewald's formula in specimens with TG
level<400 mg/dl.
Highly Sensitive C - reactive protein (hs-CRP) Assay
Highly sensitive-CRP concentrations were determined by
using latex particle enhanced immune turbid metric assay on
the Hitachi 912 auto-analyzer (Roche Diagnostic, Switzerland)
that has been standardized against the World Health
Organization reference. The normal range of hs-CRP was<3.0
mg/l (<0.03 g/l).
Insulin Assay
Fasting insulin levels were measured based on micro-particle enzyme immunoassay (MEIA) technology using Abbott
reagents with Axsym system (Abbott laboratories, Illinois, USA). All participants underwent evaluation of insulin resistance
index (IR) by using the Homeostasis model assessment
(HOMA)-formula [23]. HOMA of insulin resistance (IR) was
defined using the following formula: fasting glucose (mmol/l) x
fasting insulin (µU/ml)/22.5 [23].
Statistical Analysis
All data were expressed as median and inter quartile range,
and compared the differences between groups by using the
Mann-Whitney U-test. Spearman rank correlation was used to
assess the correlation of all clinical markers in the study
participants. Clinical variables that correlated with PAD (ABI) in
the present study were tested as independent variables by
using multivariate forward stepwise linear regression analysis.
Tests were two tailed, and a p-value<0.05 was considered
significant. All analyses were performed using the SPSS
statistical package, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
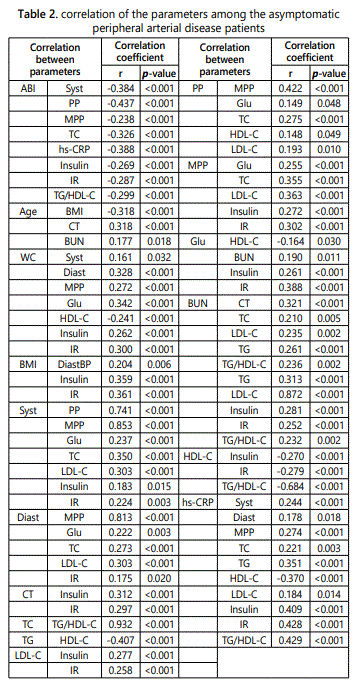
General characteristics of both groups were demonstrated in Table 1. In the comparison of clinical characteristics of both groups, Gr-1 demonstrated more difficult physical activity, significantly greater PP, TC, TG, hs-CRP, insulin, IR,TG/HDL-C ratio and lower HDL-C levels than Gr-2. Bivariate correlations, ABI was significantly correlated with Syst (r=-0.384, p<0.001), PP (r=-0.437, p<0.001), MPP (r=-0.238, p<0.001), TC (r=-0.326, p<0.001), hs-CRP (r=-0.388, p<0.001), insulin (r= -0.269, p<0.001), IR (r=-0.287, p<0.001) and TG/HDL-C ratio (r=-0.299, p<0.001). The correlation of the other clinical variables was shown in Table 2. We used multiple forward stepwise linear regression analysis to examine effects of variables in the association of these variables with ABI. Statistics were listed in Table 3. Hs-CRP, PP, TG/HDL-C ratio, IR and age showed the association with ABI, which remained highly significant after adjusting for any clinical or laboratory confounding variables [hs-CRP (β = -0.488, R2 = 0.238, p<0.001), PP (β = -0.320, R2 = 0.336, p<0.001), TG/HDL-C ratio (β = -0.279, R2 = 0.397, p<0.001), IR (β = -0.143, R2 = 0.415, p<0.001) and Age (β = -0.115, R2 = 0.428, p<0.001)].

Discussion
The risk of atherosclerotic disease is markedly increased among individuals with diabetes, hypertension and aging. Atherosclerosis is the major cause of the death and disability in these subjects [24], PAD disease is a common evidence of atherosclerosis that share the common risk factors. General characteristic of PAD is usually caused occlusive arterial vessels of the lower extremities, but many are asymptomatic [25]. The loss of function and long-term disability progression reduced walking speed and distance associated with intermittent claudicating [9, 26], subjects with low ABI<0.9 were a sign of severe atherosclerosis in their arms and legs. Our data supports these PAD patients showing increased PP, TC, TG, TG/HDL-C ratio, hs-CRP, insulin, IR, reduced HDL-C and older age.PAD (low ABI≤0.9)patients were demonstrated higher hs-CRP; an inflammation marker, is a major component and plays the major role in atherosclerosis [10,27]. Increased PP may indicate PAD patients are at high risk in for morbidity and mortality from CVD. Pulse pressure reflects vascular stiffness of the aorta and large arteries and pulse wave velocity that may be cause by atherosclerosis and aging [28]. An increased PP is associated with the development of left ventricular dysfunction and clinical heart failure in the hypertensive and elderly [29]. PAD (low ABI≤0.9) patients were also demonstrated insulin resistance state, decrease insulin function and insulin ability to inhibit lipolysis leads to increase FFAs generation and decrease lipoprotein lipase activity. This generates a chylomicron remnant rich in TG [30], caused elevated hepatic FFAs and VLDL TG-rich particles secretion. These processes also generate HDL particles containing high TG concentrations. This HDL-C high TG containing is hydrolyzed with hepatic lipase to produce TG and smaller HDL that less antiatherogenic activity and easily to remove from the body by the kidney and caused higher TG/HDL-C ratio. Thus, both insulin resistance and dyslipidemia associated with endothelial damage led increased risk of CVD [31] in these PAD (low ABI) patients. Our present study demonstrated the same as Lee et al. [32] reported association of ABI and the development of diabetic retinopathy, similar to PAD, and the study of Subramaniam et al. [33] demonstrated ABI measurement as a marker for CVD assessment in multiethnic. Previous studies have been demonstrated PAD patients or abnormal low ABI was associated with enlarge of plaques in aortic arch [34], arterial stiffness (increased PP) and aortic calcification [35].
PAD is often referred to as an under-diagnosed and under-treated public health problem. There are the practice guidelines from the American College of Cardiology/ American Heart Association for the management of PAD patients. They recommend all asymptomatic adult age ≥50 year-olds to measure ABI, especially those with current or history of smoking, diabetes and hypertension, also in adults with lower extremity circulation problems and age ≥70 years old should be assess for early CVD prevention and treatment [2]. ABI measurement is a non-invasive test for atherosclerosis detection. Therefore, routine screening for PAD has been advocated in these adults using ABI measurement. Limitations of the present study were use of cross-sectional data, a relatively small patient sample size and one district. Subjects had only one time ABI measurement.
Conclusion
ABI (or PAD) measurement should be considered as the tool for CVD risk assessment and atherosclerosis risk marker. The ABI measurement is a simple, cheap, noninvasive and reliable as the tested tool and could help the clinicians to diagnose PAD and atherosclerosis risk.
Acknowledgement
We sincerely thank the Cardiovascular and Diabetes Prevention in Elderly Project of Faculty of Allied Health Sciences for financial support to this study. We wish to thank all co-workers for their blood collection and technical assistance. We particularly thank the patients who participated in this study and Asst. Prof. Dr. Ronald A. Markwardt, Burapha University, for his critical reading and correcting of the manuscript.
Conflict of interest
The authors have no conflict of interest to report.
References