Research Article
Substantial and Sustained HbA1c reductions in Australian Insulin Pump Services for Adults with Type 1 Diabetes. Benefit also evident for Older and High HbA1c Subjects
1NHMRC Clinical Trials Centre, University of Sydney, Camperdown, NSW, Australia
2Department of Medicine, University of Melbourne, Fitzroy, VIC, Australia
3Lake Forest College, Lake Forest, IL, USA
4Department of Endocrinology and Diabetes, St Vincent's Hospital, Melbourne, VIC, Australia
5Mercy Hospital, Werribee, VIC, Australia
*Corresponding authors: Daniel A Calandro, NHMRC Clinical Trials Centre, University of Sydney, Camperdown, NSW, Australia,
Andrzej S Januszewski, Department of Medicine, University of Melbourne, Fitzroy, VIC, Australia
E-mail: andrzej.januszewski@ctc.usyd.edu.au
Received: August 28, 2016 Accepted: October 17, 2016 Published: October 21, 2016
Citation: Calandro DA, Januszewski AS, Cuper KK, et al. Substantial and Sustained HbA1c reductions in Australian Insulin Pump Services for Adults with Type 1 Diabetes. Benefit also evident for Older and High HbA1c Subjects. Madridge J Diabetes. 2016; 1(1): 23-28.doi: 10.18689/mjd-1000104
Copyright: © 2016 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: HBA1c; Type 1 Diabetes; Insulin Pump; Exogenous Insulin.
Introduction
People with Type 1 diabetes (T1D) can choose to deliver their essential exogenous insulin by multiple daily insulin injections (MDI) or by continuous subcutaneous insulin infusion (CSII) / pump therapy. Unfortunately, cost and logistic considerations of CSII therapy still remain as barriers to many patients [1,2], with approximately 10% of Australia's ≈122,000 people with T1D using insulin pumps. Whilst flexibility and quality of life for people with T1D are extremely important, many healthcare and funding agencies regard glycaemic control as the outcome of primary importance.
A meta-analysis of randomised controlled trials, not clinical practice audits, of CSII vs. MDI reported that relative to MDI use, CSII therapy lowers HbA1c levels by a mean of 0.25% (2.7mmol/mol) in adults with T1D [3]. Published CSII outcomes in Australia include: results of a user survey by the Australian Institute of Health and Welfare (AIHW) [1], a small follow-up study of CSII use in a rural setting in Infu Systems Asia [4], an audit of one tertiary referral clinic [5], and national diabetes conference abstracts re early pump experiences [6,7]. We now report results of a quality assurance (QA) audit of CSII clinical outcomes from three linked Victorian (Australia) services with some endocrinologists, diabetes educators and dietician staff in common. These clinics are all bulk-billing (i.e. covered by the national health scheme and at no out-of-pocket cost to patients). The time-frame relates to when CSII use was not associated with real-time continuous glucose monitoring (CGM) use, which may further improve glycaemic control beyond insulin pump alone, but are self-funded.
Methods
Subjects and CSII Services
Adults with T1D who commenced CSII therapy during or after 2003 at one of three collaborating services (in an Insulin Pump Clinic in the University of Melbourne, Department of Medicine St. Vincent's Hospital; a clinic run by St. Vincent's (Public) Hospital Department of Endocrinology and Diabetes, Melbourne; and a clinic at the Werribee Mercy Hospital Diabetes Service). Follow-up was up until mid-2011. The University of Melbourne based pump clinic provides a service to other endocrinologists to initiate and stabilise adults on CSII therapy using a shared care model, in which once stabilised the patients return to the sole care of their referring endocrinologist or have an annual follow-up visit only. This service provides pre- and post-pump start support. Pump initiations as a day or overnight patient are usually performed by the St Vincent's or Werribee public hospital teams or by admission to a nearby private hospital. The two public hospital clinics provide full CSII pre-education, CSII initiation and follow-up for their own patients, who are usually seen in outpatients about every three months, and more often during the pump stabilisation phase. Consultations are an admixture of small group and individual education, with carbohydrate counting being assessed, and if necessary taught, pre-CSII initiation. For inclusion in this audit subjects had to have been on CSII therapy for at least three months.
Of the 103 T1D adults commencing CSII use adequate data for inclusion were available for 77 subjects. Twenty-six subjects could not be included due to either uncertainty about their insulin pump start date (n=6), and/or a missing age of T1D diagnosis (n=17), and/or inability to obtain both baseline and follow-up biochemical measures (n=19).
Data collected
Age, diabetes duration, weight and height and blood pressure were recorded from medical records. Laboratory tests were performed by NATA accredited public hospital or private pathology laboratories and results obtained by review of (paper or electronic) medical records, or via contact with the pathology laboratories or treating physician. Data related to hypoglycaemia and hospital admissions were not collected.
Statistics
Data were recorded in MS Excel (2007) and de-identified data analysed to provide descriptive statistics. Statistical significance was taken at p<0.05. The nadir (lowest HbA1c since CSII commencement), time taken to achieve it and first time at or since CSII commencement that a patient achieved a HbA1c level below 7% were identified for each patient. The mean annual HbA1c for each patient was calculated from his or her HbA1c values measured each year post-CSII initiation. The mean value for the entire group or subgroup for each year was then calculated as the mean of the individual means. Using the same (annual mean of available measures) method we calculated the annual means for blood lipids, urinary albumin/creatinine ratio (ACR). As they were measured more frequently we calculated blood pressure and weight (measured at the clinics) at 4-monthly intervals for up to two years (as data were sparse thereafter). For comparisons of continuous variables between two groups unpaired t-tests were utilized, and ANOVA with Tukey post-hoc tests.
Results
Subjects
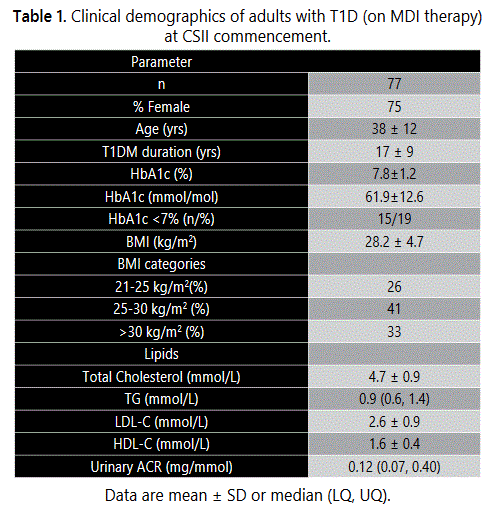
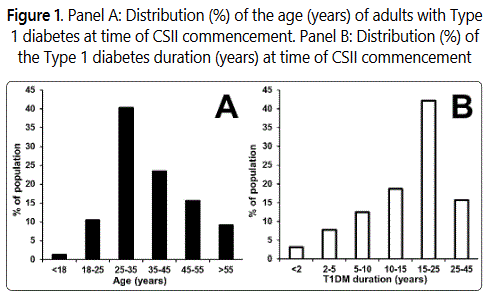
Clinical and biochemical characteristics are shown in Table 1. Baseline characteristics of the 26 subjects for whom insufficient data were available to enable inclusion in the audit did not differ significantly from the 77 subjects described in Table 1. There was a wide range of age and T1D duration at the time of CSII commencement (Table 1 and Figure 1), and there were more female than male subjects commencing CSII. The group was relatively free of vascular complications, with only two patients having increased albuminuria, and four subjects having proliferative diabetic retinopathy. No subjects had known cardiovascular disease.
All but three subjects (who used a twice daily insulin injection regimen) were using a basal bolus MDI regimen pre-CSII therapy, usually basal flat profile insulins of Lantus or Levemir and a rapid acting insulin such as Novorapid or Humalog or Apidra. Whilst not recorded for each subject, in our clinic the average daily insulin dose reduction for CSII therapy is approximately 20 - 25% from that used when on MDI. All insulin pumps were acquired via private health insurance. Common indications for CSII therapy were to improve glycaemia, including reducing risk of severe hypoglycaemia, for some women to improve glycaemia pre-pregnancy, and to improve lifestyle flexibility. All subjects obtained their pump consumables via the Australian National Diabetes Services Scheme (NDSS). No subject was regularly using continuous glucose monitoring.
HbA1c
There were no statistically significant differences in CSII benefit based on HbA1c levels by gender.
Greater HbA1c reductions in CSII subjects starting with higher HbA1c levels
The mean±SD HbA1c for the entire group fell from 7.8±1.2% (62±13mmol/mol) to a nadir of 6.7±0.8% (50±9mmol/mol), p<0.001, over a mean±SD of 17± 20 months. In contrast, in a reference group of 49 MDI users (from the same clinical services) of similar age, sex, BMI and diabetes duration, mean±SD HbA1c over 39.7±31.7 months did not change significantly from 8.0±1.4% (64±15mmol/mol) to 7.8±1.2% (62±13mmol/mol), p=0.20).
The subjects HbA1c response with CSII therapy was evaluated based on their starting HbA1c level. There was a negative univariate correlation between pre-CSII HbA1c level and the HbA1c drop at the HbA1c nadir (r=-0.76; p<0.0001). There was also a positive univariate correlation between pre-CSII HbA1c level and the value of the HbA1c nadir (r=0.42, p<0.0001), both regardless of the time the nadir was reached. As shown in Figure 2, there were significant relationships between the tertile of HbA1c at CSII commencement and the size of HbA1c levels reduction.
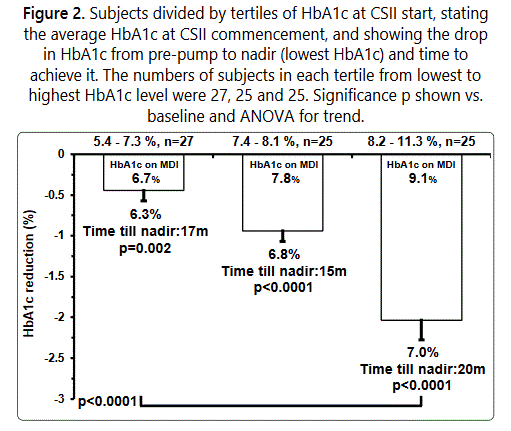
As shown in Figure 2, the higher the mean HbA1c at CSII commencement, the larger the drop in HbA1c (p<0.0001), with all groups reaching similar HbA1c nadirs, ranging between a mean of 6.3% (45mmol/mol) and 7.0% (53mmol/mol).The highest HbA1c group tended to take longer to reach their HbA1c nadir, but the time differences were not statistically significant.
Similar HbA1c reductions on CSII across age groups
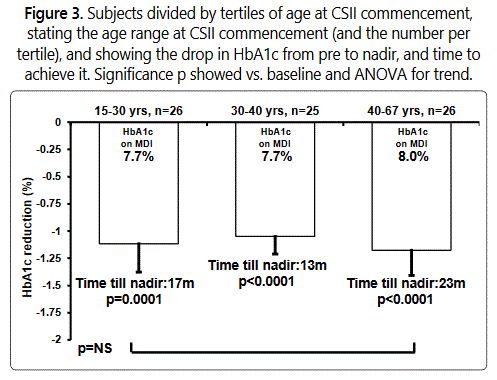
As shown in Figure 3, all tertiles based on patient age at CSII initiation dropped their HbA1c levels significantly from baseline.
There were no statistically significant differences in age related starting HbA1c level, HbA1c reduction, nadir HbA1c or time taken to achieve HbA1c nadir, though the oldest group tended to take longer to reach their HbA1c nadir.
Inverse relationship between diabetes duration and HbA1c reduction with CSII
Whilst there was not major difference in HbA1c improvement related to gender and age at CSII commencement T1D duration was related to HbA1c benefit. Participants with shorter T1D duration (≤ 12 years at CSII commencement) achieved a larger drop in HbA1c (1.8 ± 1.4%) vs. those with intermediate T1D duration (12-20 years) (0.9 ± 0.8%, p=0.007) vs. those with longer (>20 years)T1D duration (1.0 ± 1.0%, p=0.02).
Achievement of HbA1c<7% (53mmol/mol)
Pre-CSII use, only 19% of subjects had a HbA1c<7% (53mmol/mol), whereas 69% achieved an HbA1c<7% (53mmol/mol) on CSII at least once during follow-up. The percent with HbA1c<7% (53mmol/mol) at years one through four on CSII therapy were 40%, 44%, 40%, 46% (in 73, 57, 48 and 39 subjects) respectively, which was significantly greater than that at baseline. At baseline 16 subjects had HbA1c<7%. The number reaching this HbA1c target for the first time at year 1 and year 2 were 21 and 3 respectively. The number achieving HbA1c<7% for the first time in years 3 and 4 were only one each year. Only two new subjects reached this target in year 5, only one in year 6 and none reached this target for the first time in years 7 and 8.
HbA1c levels over time on CSII
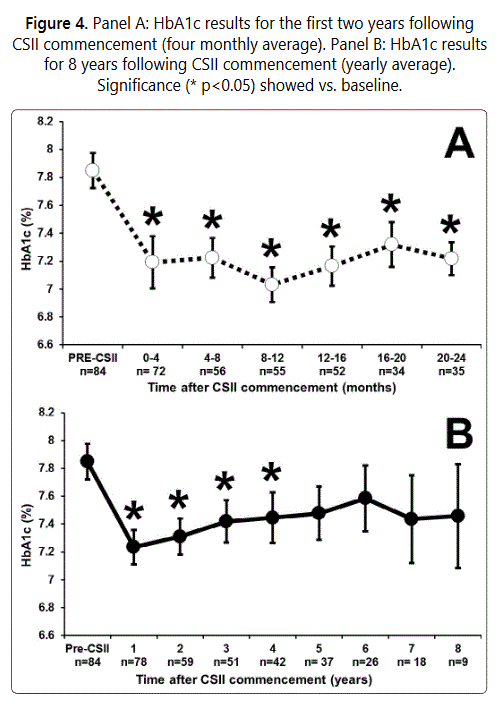
Mean HbA1c of the group pre-CSII commencement and for the first two years after CSII initiation and for up to eight years, are shown in Figure 4.
Panel A shows the mean group HbA1c data for the first two years at ≈4-month intervals post-CSII commencement, with the lowest HbA1c being at 8-12 months. Panel B shows the data at annual intervals for up to eight years post-CSII commencement. The lowest annual HbA1c (relative to MDI therapy) for the whole group was at year 1, a mean fall of 0.6% (7mmol/mol) from pre-CSII levels. Mean HbA1c levels tended to increase thereafter, with the difference (albeit with lower subject numbers) at year 5 and afterwards being lower, but not statistically significantly different, from that prior to CSII commencement.
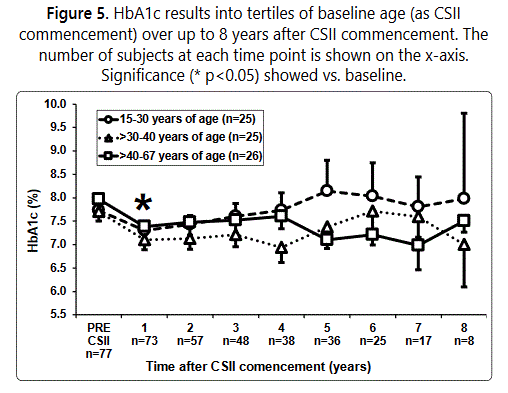
Durability of HbA1c reduction over time by age group
When mean annual HbA1c levels post-CSII were compared based on age group (shown in Figure 5). There were no significant differences between age groups.
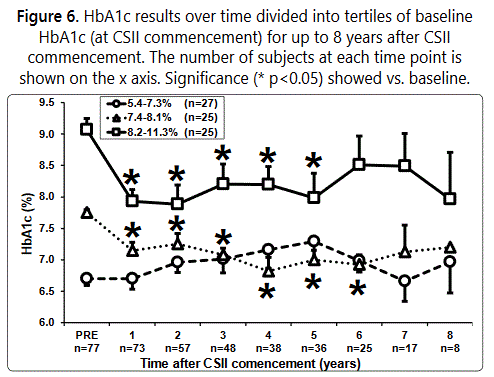
Durability of HbA1c reduction over time by starting HbA1c level
The changes in HbA1c over time when subjects were divided by baseline HbA1c tertile are shown in Figure 6. Subjects with the highest starting HbA1c tended to have higher HbA1c levels over time, but these differences did not reach statistical significance
Other clinical variables
Weight, blood pressure, lipid levels, and urine ACR (described pre-CSII therapy in Table 1) did not change significantly over follow-up. (Data not shown). Only two subjects had increased albuminuria pre-CSII therapy and we are unable to report their change over time as follow-up urine ACR data were not available.
Discussion
In three linked Victoria, Australia diabetes services, 77 subjects, predominantly females, had adequate clinical and biochemical data from pre- and post-CSII initiation for (a paper-based) Quality Assurance audit. There was a wide range of age, T1D duration and HbA1c levels at CSII initiation. Whilst there were no major differences in HbA1c improvement related to gender or age at CSII commencement, diabetes duration and starting HbA1c were associated with the subsequent change in HbA1c levels. Mean±SD HbA1c levels prior to CSII commencement was 7.8±1.2% (62±13mmol/mol), with only 19% of subjects on MDI achieving the recommended HbA1c target of less than 7% (53mmol/mol) on MDI. During follow-up (range 0.33-8) years on CSII, the HbA1c nadir was 6.7±0.8% (50±9mmol/mol), within the recommended target HbA1c<7% (53mmol/mol), achieved a mean of 17 months after CSII commencement. Over 40% of subjects achieve and maintain a HbA1c<7% (53mmol/mol) for up to four years and 69% achieve a HbA1c<7% (53mmol/mol) at least once during follow-up. Most subjects first reached a HbA1c<7% within the first 1 or 2 years of CSII commencement. With longer duration of CSII use, HbA1c levels tended to rise from the nadir, but remained significantly below pre-CSII levels for four years. Whilst all tertiles of starting HbA1c reached a nadir HbA1c of 7% (53mmol/mol) or below, the subjects with higher HbA1c levels on MDI achieved larger HbA1c reductions on CSII therapy than those with better HbA1c levels at CSII commencement. Participants with shorter T1D duration (≤ 12 years at the time of CSII commencement) also achieved a larger drop in HbA1c than those with longer diabetes duration. This may relate to lower rates of impaired hypoglycaemia awareness in those with shorter T1D duration, though we did not assess this.
The majority of patients were using MDI therapy, usually with long acting relatively peak-less insulins (Lantus or Levemir) once or twice a day, with a rapid acting insulin (Novorapid or Humalog or Apidra) pre-meal. Their mean HbA1c (7.8% (62mmol/mol)) on MDI therapy was slightly better than the national average of 8.1% (65mmol/mol) for Australian adults with Type 1 diabetes[8]. None of our subjects discontinued CSII, in keeping with low national rates of pump cessation (about 1.5%) [1].
According to Australian reports (AIHW, 2012) based on NDSS data, almost half of CSII users are below 25 years of age [1]. As reflected by Table 1 and Figure 1 we had a wide age range of subjects commencing CSII therapy, with a mean diabetes duration at CSII commencement of 17 years, with a wide range (1 - 43 years), with few (4.7%) starting CSII within the first two years of their diabetes diagnosis. We recommend that clinicians do not limit their consideration or recommendation of pump therapy to their younger diabetes patients as our data and experience support that older subjects achieve similar glycaemic outcomes.
Whilst lower HbA1c levels are associated with lower risk of diabetic vascular complications [9], reduced risk of infection [10], improved wound healing [11] and better intellectual function and mental well-being [12], it should not be the sole reason for CSII use or for judging its benefit. In a survey of CSII users in Australia 88% chose CSII to improve glucose control, and 67% also desired it for better lifestyle and quality of life [1]. We did not survey quality of life in our subjects, but their ongoing use of CSII suggests their balance of benefits and advantages of CSII use were favourable. In other pump studies, including our own, CSII use was associated with improved quality of life [13-18].
In our clinical audit subjects achieved a mean HbA1c reduction of 1.1% (12mmol/mol), with more than a doubling of subjects achieving a HbA1c<7% (53mmol/mol), the generally recommended target in clinical practice, however we acknowledge that HbA1c targets should be individualised [19]. For example, for elderly people, patients with reduced hypoglycaemia awareness, or those living alone, a higher HbA1c target may be prudent. On average the HbA1c levels and reductions observed are greater than those reported in recent studies [3,20]. In Sweden 10 clinics conducted a similar audit on patients with Type 1 diabetes with a mean HbA1c of 8.4% (68mmol/mol) and reported a mean HbA1c reduction of 0.5% (5mmol/mol) after 1-year of CSII use [20]. These differences likely reflect patient selection and differences in clinical practice. That all subjects in our study reached similar HbA1c nadirs irrespective of their starting HbA1c level may reflect patient preference so as to limit their hypoglycaemia risk and frequency, which we did not record due to challenges in the accuracy of data collection, effects of impaired hypoglycaemic awareness, which is not uncommon in T1D, and the absence of continuous glucose monitoring use.
Analysis of our patients' HbA1c results by tertiles of age at CSII commencement revealed similar HbA1c benefit between age groups, with similar time taken to reach their HbA1c nadir. Similarly, Matejko et al. reported that CSII users aged≥50 years, achieved similar HbA1c benefit as those <50 years [21].Our outcomes support that adults with T1D of all ages should be considered for CSII therapy. We did find that shorter T1D duration subjects achieved greater HbA1c reductions with CSII use, which may relate to less impaired hypoglycaemia awareness, which is more likely with longer diabetes duration, but we did not record hypoglycaemia awareness. Our results and that of other investigators, such as Matejko, dispel the not uncommon notion that youth perform better than more mature adults with regard to glycaemic benefit with pump use in spite of differences in the hormonal milieu and lifestyle. However, we recognise that self-selection bias is present. Our audit is not able to discern the effects of education and time spent with the diabetes care team that would have been given to those subjects starting CSII therapy, nor assess whether the clinician time spent differed between age groups or by starting HbA1c level.
The weight, blood pressure and lipid levels of our patients did not change significantly over time. The average lack of weight change with CSII use is in keeping with prior reports. [22]. Whilst the reduction in insulin dose on CSII vs. MDI therapy may be associated with reduced appetite, others may gain weight due to the greater flexibility of food intake and lower HbA1c reducing (calorie losing) glycosuria. Whilst better glycaemic control is usually associated with improved lipid levels, there were no significant changes. We did not fully record use of and/or changes in diet and lipid or blood pressure medications. Dahl-Jørgensen >et al reported reductions in albuminuria with CSII use [23], likely related to better glycaemia. There was no change in ACR in our subjects, though all but two had normal levels, and we acknowledge that the urine ACR is a screening test. Epidemiologic studies, such as the Swedish Diabetes Registry v] have demonstrated lower vascular complication and mortality rates with CSII use than with MDI use, which may relate to better HbA1c levels, and potentially (not reported) also to lower glycaemic variability and lower exogenous insulin doses.
Study limitations include incomplete follow-up of all subjects commencing CSII. Since undertaking this audit an electronic data-base has been adopted, so completeness and follow-up of future audits will likely be better and less labour intensive. It is likely that the CSII users received more time and education for the clinicians than those subjects continuing on MDI. We did not record clinician time to achieve the CSII-related improved glucose control, but in a subsequent prospective study we demonstrated that it is significantly longer for nurses, but similar for doctors and dietitians to support CSII than MDI users [2]. As is common in our clinic and in that of many of our colleagues, the majority of our pump users were female; hence the relatively small number of males evaluated does not enable robust comparison of potential gender differences in responses. Similarly, very few subjects had chronic diabetes complications; hence we are unable to discern if their metabolic responses differed and if this had any effect on their chronic complications. As this is an audit of clinical practice rather than a randomised controlled trial, there may be a (self-) selection bias of pump users being more motivated or more affluent than the general population of adults with Type 1 diabetes. A strength is that this audit represents 'real-world' clinical practice at different clinical sites and with several years of follow-up.
In conclusion, we have demonstrated in our clinical practices that adult men and women with Type 1 diabetes across a wide range of age, diabetes duration and initial HbA1c levels do well with CSII use, with sustained HbA1c reductions and no changes in weight, blood pressure, lipids or urine ACR over time. HbA1c reductions were greater in those starting CSII therapy with the highest HbA1c level and with shorter diabetes duration, and were similar and well-maintained in all age groups.
Acknowledgements: Authors thank all medical clinic, hospital and pathology laboratory staff for assistance with data retrieval and their input into patient care.
Authors Disclosure Statement: KKC was supported by a travel grant from Global Links, USA. AJJ and DNO have received honoraria from and conducted (investigator and industry initiated) trials from Medtronic and Sanofi-Aventis, Animas, Novo and Eli Lily. AJ is on Advisory Boards for Medtronic and Abbott and DNO is on an Advisory Board for Abbott.RJM has received honoraria for lectures from Eli Lilly, Novo Nordisk, Sanofi-Aventis, Astra Zeneca, Merck Sharp & Dohme, Bayer and Norvartis and travel support from Novo Nordisk, Sanofi-Aventis, Boehringer Ingelheim and Astra Zeneca. He has received research grants from Novo Nordisk and Servier, conducted industry initiated trials for Novo Nordisk, Bayer, Johnson & Johnson, Astra Zeneca, Abbive and Versartis and on the Advisory Board for the Boehringer Ingelheim - Eli Lilly Alliance and Sanofi-Aventis.
Conflicts of Interest: The authors report no conflicts of interest.
References