Research Article
Part III: Dentinogenic Effect of Magnesium Oxide on BMP2/SMAD Signaling Pathways in Normal Human Dental Pulp Cells: An In Vitro Study
1Department of Restorative Sciences & Biomaterials, Goldman School of Dental Medicine, Boston University, Boston, MA 02118, United States
2Department of Endodontics, Goldman School of Dental Medicine, Boston University, Boston, MA 02118, United States
*Corresponding author: Rania M Salem, Department of Restorative Sciences & Endodontics, Goldman School of Dental Medicine, Boston University, USA, Tel: +3474093109, E-mail: rmsalem@bu.edu
Received: March 21, 2022 Accepted: April 06, 2022 Published: April 12, 2022
Citation: Salem RM, Zhang C, Chou L. Part III: Dentinogenic Effect of Magnesium Oxide on BMP2/SMAD Signaling Pathways in Normal Human Dental Pulp Cells: An In Vitro Study. Madridge J Dent Oral Surg. 2022; 7(1): 127-132. doi: 10.18689/mjdl-1000126
Copyright: © 2022 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Magnesium enriched microenvironment might create a favorable milieu conducive to dentin repair and/or regeneration. In previous work, magnesium oxide (MgO) has been considered for its potential ability to enhance cell attachment, proliferation rate and expression of dentin matrix proteins. However, to date the role of MgO and the involved regulatory mechanisms of the BMP/Smad pathway in mediating odontoblastic differentiation remain undefined. This study was designed to determine and compare the stimulatory effect of different concentrations of MgO on expression of bone morphogenic protein (BMP-2) and SMADs 1/5/9 in signal transduction pathway of normal human dental pulp cells (HDPCs). HDPCs were cultured with 0.5 mM, 1 mM, 2 mM, 4 mM, 8 mM concentrations of supplemental MgO, 0 mM as the negative control group, lignin sulfonic acid sodium salt and xanthan gum as the vehicle control groups. Statistical analysis using Multi-Way Analysis of Variance (MANOVA) with Wilksʼ lambda test. Results showed that 0.5 mM MgO elicited the highest stimulatory effect on expression of BMP-2 and SMADs 1/5/9 compared to other concentrations of MgO and the negative and vehicle control groups at all time points (P<0.001). Supplemental MgO concentrations higher than 0.5 mM had an inhibitory effect on HDPCs with lower expression of BMP-2 and SMADs 1/5/9 compared to the control groups (P<0.001). Conclusively, this is the first study to reveal that MgO at an optimal concentration (0.5 mM) might promote the differentiation of HDPCs in to odontoblast-like cells through the activation of BMP/SMAD signaling pathway. This study may provide a new insight that MgO might be considered for new therapeutic opportunities in regenerative endodontics.
Keywords: Magnesium oxide; Bone morphogenic protein; SMADs; Regenerative endodontics.
Introduction
Dental caries remains the threat to the dental pulp [1], resulting from the penetration of oral bacteria into the enamel and dentin. In the presence of carious lesions, pulpal exposure by cavity preparation, and following restorative procedures, a wound healing process occurs. Surviving odontoblasts and odontoblast-like cells differentiate from progenitor stem cells in the dental pulp in a highly coordinated interplay of different growth factors and cytokines to form reparative dentin required to block further noxious stimuli [2-5]. Both pulp repair and regeneration processes are orchestrated by these key regulatory factors. Bone morphogenic proteins (BMPs) are the most widely studied growth factors involved in differentiation of osteoblasts and odontoblasts.
BMP-2 and its intracellular transducers such as SMAD1 and SMAD 5 serve as regulators of tooth development [6]. BMP2 activates intracellular signaling by binding to heterooligomeric serine/threonine kinase receptor complexes composed of type I and type II BMP receptors presented on the cell surface [7]. After ligand binding autophosphorylated BMP type II receptors phosphorylate type I receptors, which in turn activates canonical signaling via phosphorylation of SMADs 1/5/9. It was reported that BMP-2 induced the phosphorylation and nuclear translocation of SMADs 1/5 mediating odontoblastic differentiation in dental pulp cells (DPCs) [8]. Taken together, BMP/SMAD signaling pathway might play an important role during reparative dentin formation.
Previous studies showed that MgO upregulated attachment, proliferation rate, alkaline phosphatase (ALP) activity, and expression of dentin sialoprotein (DSP), dentin matrix protein (DMP-1), dentin sialophosphoprotein (DSPP) and type I collagen (COL-I) in HDPCs in vitro [9,10]. However, the precise molecular mechanisms of MgO responsible for odontoblast differentiation are not fully investigated. In the present study, the main objective was to evaluate the stimulatory effect of different concentrations of MgO on expression of BMP-2 and SMADs 1/5/9 and determine the associated signaling pathways of HDPCs in vitro. This is the first report to show a MgO-stimulated effect in HDPCs that may lead to the upregulation of BMP-2/SMAD signaling in vitro.
Materials and Methods
Preparation of supplemented MgO suspension
An aqueous MgO suspension was prepared using MgO powder milled size ranging from 44 to 297 micron (Mag Chem 10, Martin Marietta Magnesia Specialties, Baltimore, MD, USA) based on the combined weight of water, lignin sulfonic acid sodium salt (anti-hydration agent) and xanthan gum (suspension aid), providing a highly stable concentrated suspension (according to the patent developed by Richard Van De Walle 1989) [11]. Five stock solutions were prepared at concentrations of 5 mM, 10 mM, 20 mM, 40 mM and 80 mM respectively.
Lignosulfonic acid sodium salt and xanthan gum solvent preparation
Lignosulfonic acid sodium salt and xanthan gum (Sigma Aldrich, MA, USA) were dissolved in deionized water and subsequently filtered under sterile condition in the biological hood, kept in a labeled container and stored at room temperature, utilized as vehicle controls to be compared to the negative control.
HDPCs primary culture
Human dental pulp explants were obtained from extraction of impacted third molars through the oral surgery department at Boston University School of Dental Medicine. Healthy patients with an age range of 15-25 years were screened to rule out any metabolic or systemic disease, acute infections, or any steroid drugs that were taken in the last six months preceding the surgery. Exclusion criteria included caries, restorations, trauma, non-vital teeth or teeth associated with pathologic conditions. This study was approved under Boston University IRB approval H-33173. HDPCs were isolated following a previously published protocol with modifications [9,10,12]. First, sagittal indentation was made using a high-speed handpiece and fissure bur with a coolant without exposing the pulp. Sectioning was carried out using a #7 Chandler bi-bevel bone chisel (Hu-Friedy, Chicago, IL, USA) to expose the pulp. The pulp tissue was divided into small pieces and were placed in 12.5 cm2 culture flasks (Thermo Fisher Scientific, Cambridge, MA, USA). Culture medium consisted of 10% fetal bovine serum (FBS) (R&D Systems, Minneapolis MN, USA), 1X Penicillin antibiotic (100 U/mL), and 1X Streptomycin (100 ug /mL) (Gibco, MA, USA), Amphotericin B anti-fungal (0.25 ug/ml) in Eagleʼs Basal Medium (BME) (Gibco, MA, USA). The cultureflasks were maintained in an incubator at 37°C C with 5% CO2 and saturated humidity and cultured up to the second passage. Growth media was replaced every 72 hours.
When nearly confluent, cells were trypsinized with 0.05% Trypsin (Gibco, MA, USA). Cells were counted using a hemocytometer and used in the experiments. Characterization of dentinogenic phenotype of the cells was confirmed by expression of dentinogenic markers induced by vit D3 stimulation as previously reported [9,10]. HDPCs were then transferred to 24 well plates (Thermo Fisher Scientific, MA, USA) and grown in the culture medium supplemented with 0mM (control), 0.5 mM, 1 mM, 2 mM, 4 mM and 8 mM supplemental MgO concentrations respectively. For cellular differentiation studies, growth media were replaced with pre-inductive dentinogenic media with 10 nM Vitamin D3 (172g/mol) (Sigma Aldrich, MA, USA) for two additional days before the predetermined time intervals of 4, 7, and 11days. Dentinogenic media was composed of the following: 10% charcoal stripped fetal bovine serum (FBS) (Life Technologies, MA, USA), 100 IU/ml Penicillin (Gibco, Waltham, MA, USA), 100 µg/m streptomycin (Gibco, MA, USA), 10-8M Menadione (Sigma Aldrich, Burlington, MA, USA), 10 mM β-Glycerophosphate (Sigma Aldrich, Burlington, MA, USA), 0.05 mg/mL L-ascorbic acid (Sigma Aldrich, MA, USA), 2 mM L-glutamine (Gibco, Waltham, MA, USA). From measurements obtained from proliferation data in previously published study [9], the cell numbers for each time interval 7, 10 and 14 days were used to determine the values of extracellular protein level on per million cell base formula.
Assessment of BMP-2 expression in HDPCs
Cell culture supernatant samples at days 7, 10 and 14 were tested using BMP-2 Xpress BioTM ELISA Kit (Human), to evaluate expression levels of secreted BMP-2 protein. In a 96- well microplate (Thermo Fisher Scientific, MA, USA), five replicates were tested for each of MgO concentrations: 0.5 mM, 1 mM, 2 mM, 4 mM, 8 mM and 0 mM as the negative control, and lignin sulfonic acid sodium salt and xanthan gum as the vehicle control. Supernatants with various conditions were centrifuged at 1000 xg for 20 minutes to remove cellular debris. Microtiter plate was washed twice with wash buffer before adding standards, sample, and controls (blank wells). The standard solutions were prepared in concentrations of 7.8 pg/mL, 15.625 pg/mL, 31.25 pg/mL, 62.5 pg/mL, 125 pg/mL and 250 pg/mL. Sample dilution buffer was aliquoted and added into the standard and the control (blank) wells. Microtiter plates with 100 µL of the diluted (2x) test samples, standards and blanks were incubated at 37°C for 90 minutes. The plate contents were then discarded and washed twice with wash buffer. 100µL of the biotin conjugated antibody was added subsequently in the antibody pre-coated microtiter plate. The biotin conjugated antibody solution was added at the bottom of each well. The plate was then sealed with a cover and incubated for 60 minutes at 37°C. After the plate was washed for three washes, 100 µL horseradish peroxidase streptavidin (HRP-Streptavidin) was added and incubated for 30 minutes. Unbound conjugate was washed away five times with wash buffer. 90 µL TMB substrate was then added into each well followed by incubation in dark for 10-20 minutes. Finally, the HRP enzymatic reaction was terminated by adding 50 µL stop solution. The intensity of color (O.D) versus the respective BMP-2 concentration in the sample or the standard was identified with microplate reader (TECAN, Infinite 1000 Pro) at a wavelength of 450 nm. BMP-2 concentration was calculated by a standard curve and normalized to BMP-2 expression measured per million of cells.
Assessment of SMADs 1/5/9 signaling protein expression in HDPCs
Qualitative determination of target protein concentration of SMADS 1/5/9 in cultured cells was achieved by using a colorimetric cell-based indirect ELISA Kit format CytoGlow™ (Assay Biotechnology, USA). A monoclonal antibody specific for human glyceraldehyde -3- phosphate-dehdrogenase (GAPDH) was included to serve as an internal positive control in normalizing the target absorbance values. HDPCs were seeded at a density of 20,000 cells per well in 200 µL of culture medium in a 96-well plate (Thermo Fisher Scientific, MA, USA). Five replicas were tested for each of various concentrations of supplemental MgO: 0.5 mM, 1 mM, 2 mM, 4 mM, 8 mM, 0 mM as negative control, lignin sulfonic acid sodium salt and xanthan gum as vehicle controls respectively. SMADs 1/5/9 protein expression levels were measured at 16 hr, 7, 10 and 14 days. The culture plates were incubated at 37°C and 5% CO2. Cell culture medium was removed and rinsed with 200 µL of 1x TBS twice. Cells were then fixed in 100 µL of 4% formaldehyde for 20 min at room temperature and washed three times with 200 µL 1x wash buffer for 5 min each time. 100 µL of quenching buffer was added for 20 min at room temperature and washed with 1x wash buffer for 5 min. Then, 200 µL of blocking buffer was added for 1 hr at room temperature. After washing with 200 µL 1x wash buffer, 50 µL of 1x primary antibody (Anti-SMADs 1/5/9) was added. Anti-GAPDH antibody was added to the positive control wells. After incubation for 16 hrs at 4°C, plates were washed three times with 200 µL 1x wash buffer for 5 min. Then, 50 µL of secondary antibody (HRP- conjugated Anti-rabbit IgG) was added to the wells containing the Anti-SMADs 1/5/9 and to the negative control wells. The secondary antibody (HRP-conjugated Anti-Mouse IgG) was added to the positive control wells (Anti-GAPDH antibody) for 1.5 hr at room temperature. After washing with 200 µL of 1x wash buffer, 50µL of ready to use substrate was added to each well for 30 min at room temperature in dark, then 50 µL of stop solution was added. The ELISA plates were read using the microplate reader at 450 nm for HRP activity. After measuring absorbance at 450 nm, plates were washed twice with 200 µL of 1x wash buffer, and then twice with 200 µL of 1x TBS. Plates were tapped on paper towels to remove excess liquid, then, were left to air dry at room temperature. Then, crystal violet staining method was used to determine optical cell density number. 50 µL of crystal violet solution was added for 30 min at room temperature, followed by rinsing with 100 µL of distilled water for four to five times till the wells became transparent drying for 30 minutes, and adding 100 µL of SDS for 1 hr. Absorbance was read with the microplate reader (TECAN, Infinite 1000 Pro) at 595 nm. The results were analyzed by normalizing the absorbance values to cell numbers. OD values at 450 nm obtained for the SMADs 1/5/9 target proteins were normalized with the OD values at 450 nm obtained for GAPDH. For crystal violet staining normalization, the measured OD values at 450 nm were normalized with the OD values at 595 nm by dividing OD 450/OD 595.
Statistical Analysis
The means and standard deviations (SD) data of the levels of BMP-2 expression at 7, 10, and 14 days, and SMADs 1/5/9 expression levels at 16 hours, 3, 7, 10 and 14 days, were calculated and are presented in Tables (1-4). The data were normalized on a per million cells basis at the same time points. Statistical analysis was performed using software JMP Pro 13 (ver. 13.1.0). Multi-Way Analysis of Variance (Multi-Way ANOVA) with Wilksʼ lambda test was used for statistical analysis between the groups. Differences at P≤0.05 were considered statistically significant.
Results
Effect of MgO on BMP-2 expression of HDPCs
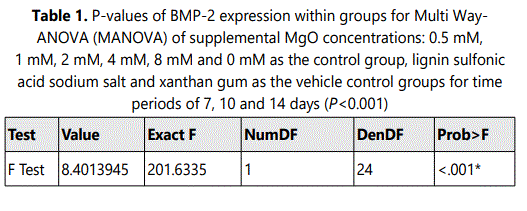
In the 0.5 mM supplemental MgO concentration groups of all time intervals a significant increase in BMP-2 expression were observed compared with the higher supplemental MgO concentration groups and all control groups (P<0.001). The supplemental MgO concentration groups higher than 0.5 mM showed significant lower BMP-2 expression comparable to the negative control and vehicle control groups (P<0.001). (Figure 1) (Table 1). Wilksʼ Lambda interaction P-value for supplemental MgO concentrations showed a statistically significant value at this time point (P<0.001) (Table 2).
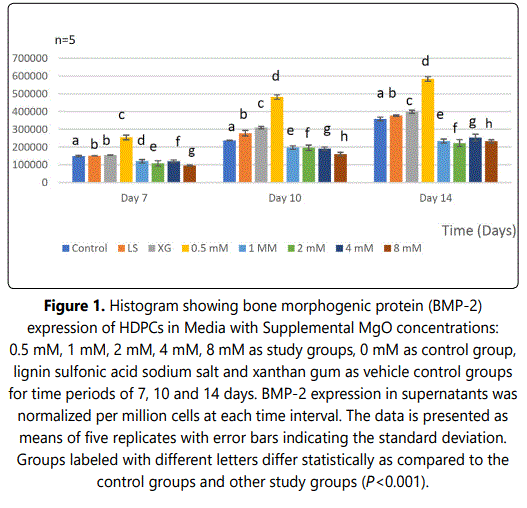
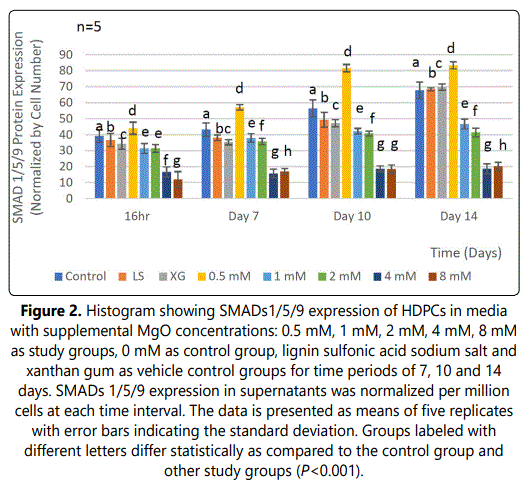
Effect of MgO on SMADs 1/5/9 expression of HDPCs
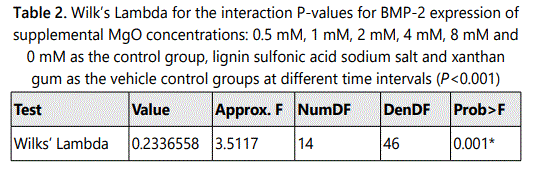
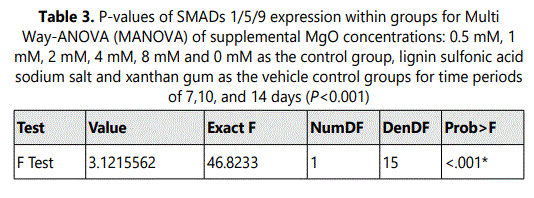
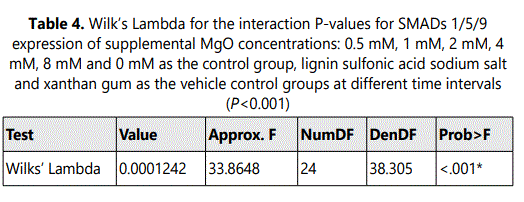
A significant increase in SMADs 1/5/9 expressions was observed at all time intervals 16 hrs, 7 days, 10 days and 14 days in the 0.5 mM supplemental MgO concentration group in comparison with higher supplemental MgO concentration groups and all control groups (P<0.001) (Figure 2) (Table 3). Wilksʼ Lambda interaction P-value for supplemental MgO concentrations demonstrated a statistically significant value (P<0.001) (Table 4). The 1 mM, 2 mM, supplemental MgO concentration groups showed significantly lower levels of SMADs 1/5/9 expressions at day 10 and day 14 cosmpared to all control groups (P<0.001). The 4 mM and 8 mM supplemental MgO concentration groups showed significantly lower levels of SMADs 1/5/9 expressions at all time intervals of 16 hrs, 7 days, 10 days and 14 days compared to all control groups (P<0.001). Wilksʼ Lambda interaction P-value for supplemental MgO concentrations pointed out a statistically significant value (P<0.001) (Table 4).
Discussion
In the present study the effect of supplemental MgO at various concentrations on expression of BMP2/SMAD involved in signaling pathways and mediating odontoblastic differentiation was assessed. BMPs are known to have diverse biological functions during embryonic development [13,14] and osteogenesis [15,16] acting through complex signaling networks [17]. Through two transmembrane receptors, type I and type II, with serine-threonine kinase activity expressed in dental pulp, BMP signals are transferred to the nucleus by SMAD proteins. BMP signaling regulates epithelial-mesenchymal interactions during tooth development and has been implicated in early mineralization stages [18]. BMP-2 is expressed in mesenchymal cells [19] and promotes their pluripotent commitment to the osteoblast lineage by regulating transcription factors such as Runx2/SMAD cascade [20,21]. BMP-2 induces ameloblast and odontoblast precursor cell differentiation in vitro [22]. Conclusively, the data demonstrated that dentin-derived BMP-2 is required to induce the differentiation of dental pulp cells into odontoblasts. In the present study, BMP-2 expression at day 7 presented a significant increase in the 0.5 mM MgO concentration group at all time intervals. These results are in accordance with Ke et al. [23] whom similarly reported higher BMP-2 expression on tricalcium phosphate/MgO (TCP-MgO) scaffolds containing 1wt% MgO, showing enhanced early osteoblastic differentiation. Similarly, Huang et al. [24] whom developed a biomimetic scaffold by incorporating CaCO3/MgO into a carboxymethyl chitosan (CMC) matrix reported that the 1% (W/V) CaCO3/MgO/CMC combined scaffolds seeded with human adipose-derived mesenchymal stem cells (hADSC) exhibited significant osteogenic differentiation potential and mineralization ability compared with CMC and tissue culture plate (TCP) groups. In a study by Xing et al. [25] BMP2 showed nearly 6.4-fold increased expression in pre-osteoblastic (MC3T3-E1) cells on silk fibroin, polycaprolactone (SF/PCL)-blend scaffolds containing MgO. The obtained Mg-functionalized scaffolds displayed satisfactory osteogenic capability dueto the controlled release of MgO [26-28]. It is believed that BMP-2 binded to their respective (BMP-2R) receptors which might have activated the transcription of the BMP-2 signaling pathway. The specific pathway that is activated upon ligand-receptor interaction is thus likely dependent upon Mg2+ enriched extra cellular microenvironment, and crosstalk with other pathways. The results of the present study showed that HDPCs with odontoblastic properties are useful to shed light on the molecular mechanisms and signaling pathways of BMP-2.
SMADs are the signaling effectors and the only TGF-β receptor substrates with a demonstrated ability to propagate signals during osteogenesis/dentinogenesis. There are 3 types of SMADs: receptor regulated (R-SMADs), common mediator (Co-SMADs), and inhibitory (I-SMADs) [29]. The type I BMP receptors specifically recognize the receptor-activated R-SMADs including SMAD2 and SMAD3, which are recognized by TGF‐β and Activin receptors, and SMADs 1/5/9, which are recognized by BMP receptors. Only R-SMADs are targeted for activation via phosphorylation by the active type I receptor kinase. Following the phosphorylation of R-SMADs (SMADs 1/5/9) by BMPs, heteromeric complexes are formed with Co-SMAD (SMAD 4) and translocate to the nucleus where they regulate the transcription of target genes/proteins together with other nuclear cofactors. The effects of BMP2/ SMAD signaling pathways on skeletal cells have been the topic of numerous in vitro studies [30]. Omi et al. [31] showed that loss of BMP signaling resulted in reduced crown and root dentin formationbut restoring SMAD signaling activity rescued dentin formation. Qin et al. [8] examined the involvement of the BMP2/SMAD pathway in mediating odontoblastic differentiation in DPCs. Addition of BMP-2 up-regulated phosphorylated (p)-SMADs 1/5 activity. However, when phosphorylation and nuclear translocation of SMADs 1/5 were partially inhibited by noggin, a specific inhibitor of BMP signaling, downregulation of alkaline phosphatase (ALP) activity, DSPP, DMP-1, nest in expression and less mineralized nodules were observed. Washio et al. [32] reported using odontoblast-like (KN-3) cells to elucidate SMAD signaling pathway activation, treatment of KN-3 cells with BMP-2 induced activation and expression of intracellular signaling molecules, such as SMADs1/5/9 and SMADs 6/7, as well as DSP and DMP-1. The above forementioned studies highlightedthe role of BMP-SMAD pathway in mediating odontoblastic differentiation and dentinogenesis during pulp regeneration process.
In the present study, comparing SMADs 1/5/9 expression at different time intervals throughout the present work clearly showed the direct enhancing effect of SMADs expression with 0.5 mM MgO concentration groups on HDPCs. The results demonstrated that at 7 days 0.5 mM MgO concentration group showed an increase in the SMADs 1/5/9 expression. Moreover, at days 10, and 14 the same concentration group had a significantly higher increase in SMADs1/5/9 expression. The data of the present study is in agreement with Kongʼs report [33] whom showed that SMADs 1/5/9 protein levels were markedly elevated in DPSCs cultured in Mg2+enriched odontogenic medium, showing the same trend regarding the concentration range and the time points. Moreover, Ding et al. [34], Zhang et al. [35], corroborated that Mg2+ ions induced more expression as well as more robust phosphorylation of SMADs 1/5/9 protein of C2C12 and BMSCs cells in vitro. Considering the present study as the first to investigate the role of Mg2+ in inducing SMADs 1/5/9 expression in normal human heterogenic dental pulp cells (HDPCs), the findings from the above and present studies authenticate that normal HDPCs, DPSCs and BMSCs cells could exhibit similarities in Mg2+ induced SMADs1/5/9 expression. These studies provided new insight that MgO might have induced BMP2/SMAD signaling pathways facilitating dentinogenesis.
Conclusion
The data presented in this study suggested the beneficial effect of 0.5 MgO on promoting odontoblastic differentiation, through the activation of BMP-2/ SMAD signaling pathway. This is the first report to demonstrate the optimal MgO concentration needed to significantly enhance dentinogenic activities of HDPCs. MgO containing biomaterials could be considered to develop pertinent regeneration therapies for the dentin-pulp complex.
Conflict of Interests
The authors declare no conflict of interests.
Funding Statement
The authors declare no financial support.
Acknowledgments
The authors are grateful to the Oral Surgery Department, Boston University Henry M. Goldman School of Dental Medicine and would also like to express their deepest thanks to Richard H. Van de Walle for supporting this work.
References
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015; 94(5): 650-8. doi: 10.1177/0022034515573272
- Duncan HF, Cooper PR, Smith AJ. Dissecting dentine-pulp injury and wound healing responses: consequences for regenerative endodontics. Int Endod J. 2019; 52(3): 261-266. doi: 10.1111/iej.13064
- Smith JG, Smith AJ, Cooper PR. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp Cell Res. 2012; 318(18): 2397-406. doi: 10.1016/j.yexcr.2012.07.008
- Murray PE, Garcia Godoy F, Hargreaves M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J Endodontics. 2007; 33(4): 377-390. doi: 10.1016/j.joen.2006.09.013
- Maddaluno L, Urwyler C, Werner S. Fibroblast Growth Factors: Key Players in Regeneration and Tissue Repair. Dev. 2017; 144(22): 4047-4060. doi: 10.1242/dev.152587
- Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (BMPs) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997; 210(4): 383-96. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C
- Nohe A, Hassel S, Ehrlich M, et al. The Mode of Bone Morphogenetic Protein (BMP) Receptor Oligomerization Determines Different BMP-2 Signaling Pathways. J Biol Chem. 2002; 277(7): 5330-5338. doi: 10.1074/jbc.M102750200
- Qin W, Yang F, Deng R, et al. Smad 1/5 is involved in bone morphogenetic protein-2-induced odontoblastic differentiation in human dental pulp cells. J Endod. 2012; 38(1): 66-71. doi: 10.1016/j.joen.2011.09.025
- Salem RM, Zhang C, Chou L. Dentinogenic Effect of Magnesium Oxide on Normal Human Dental Pulp Cells: An In Vitro Study. Madridge J Dent Oral Surg. 2021; 6(1): 106-115. doi: 10.18689/mjdl-1000124
- Salem RM, Zhang C, Chou L. Part II: Dentinogenic Effect of Magnesium Oxide on Expression of Extracellular Matrix Proteins in Normal Human Dental Pulp Cells: An In Vitro Study. Madridge J Dent Oral Surg. 2021; 6(1): 116-126. doi: 10.18689/mjdl-1000125
- Raoof M, Yaghoobi MM, Derakhshani A, et al. A modified efficientmethod for dental pulp stem cell isolation. Dent Res J (Isfahan). 2014; 11(2): 244-50.
- Wang RN, Green J, Wang Z, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014; 1(1): 87-105. doi: 10.1016/j.gendis.2014.07.005
- Beederman M, Lamplot JD, Nan G, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013; 6(8A): 32-52. doi: 10.4236/jbise.2013.68A1004
- Wu M, Chen G, Li YP. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Research. 2016; 4(16009):1-21. doi: 10.1038/boneres.2016.9
- Puerto MCG, Iyengar PV, Vinuesa AG, Dijke PT, Duffhes GS. Bone morphogenetic protein receptor signal transduction in human disease. J Pathol. 2019; 247(1): 9-20. doi: 10.1002/path.5170
- Jussila M, Thelseff M. Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages. Cold Spring HarbPerspect Biol. 2012; 4(4): 1-13. doi: 10.1101/cshperspect.a008425
- Marupanthorn K, Tantrawatpan C, Kheolamai P, Tantikanlayaporn D. Bone morphogenetic protein-2 enhances the osteogenic differentiation capacity of mesenchymal stromal cells derived from human bone marrow and umbilical cord. Int J Mol Med. 2017; 39(3): 654-662. doi: 10.3892/ijmm.2017.2872
- Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007; 12(1): 3068-3092. doi: 10.2741/2296
- Afzal F, Pratap J, Ito K, et al. Smad function and intranuclear targeting share a Runx2 motif required for osteogenic lineage induction and BMP2 responsive transcription. J Cell Physiol. 2005; 204(1): 63-72. doi: 10.1002/jcp.20258
- Arakaki M, Ishikawa M, Nakamura T, Harada H, Yamada Y. Role of Epithelial-Stem Cell Interactions during Dental Cell Differentiation. J Biol Chem. 2012; 287(13): 10590-10601. doi: 10.1074/JBC.M111.285874
- Huang YZ, Ji RY, Kang ZW, et al. Integrating Eggshell-derived CaCO3/MgO Nanocomposites and Chitosan into a Biomimetic Scaffold for Bone Regeneration. Chem Eng J. 2020; 395(1): 125098-99. doi: 10.1016/j.cej.2020.125098
- Xing X, Cheng G, Yin C, Cheng X. Magnesium-Containing Silk Fibroin/Polycaprolactone Electrospun Nanofibrous Scaffolds for Accelerating Bone Regeneration. Arab J Chem. 2020; 13(5): 1-39. doi: 10.1016/j.arabjc.2020.03.031
- Kargozar S, Milan BP, Amoupour M, et al. Osteogenic Potential of Magnesium (Mg)-DopedMulti component Bioactive Glass: In Vitro and In Vivo Animal Studies. Materials. 2022; 15(1): 318-30. doi: 10.3390/ma15010318
- Prymak O, Vagiaki LE, Buyakov A, et al. Porous Zirconia/Magnesia Ceramics Support Osteogenic Potential In Vitro. Materials. 2021; 14(1): 1049-54. doi: 10.3390/ma14041049
- Lin Z, Shen D, Zhou W, et al. Regulation of extracellular bioactive cations in bone tissue microenvironment induces favorable osteoimmune conditions to accelerate in situ bone regeneration. Bioactive Materials. 2021; 6(8): 2315-2330. doi: 10.1016/j.bioactmat.2021.01.018
- Attisano L, Horflich STL. The Smads. Genome Biology. 2001; 2(3010): 1-8. doi: 10.1186/gb-2001-2-8-reviews3010
- Song B, Estrada KD, Lyons KM. Smad Signaling in Skeletal Development and Regeneration. Cytokine Growth Factor Rev. 2009; 20(5-6): 379-388. doi: 10.1016/j.cytogfr.2009.10.010
- Omi M, Kulkarni AK, Raichur A, et al. BMP-Smad Signaling Regulates Postnatal Crown Dentinogenesis in Mouse Molar. JBMR Plus. 2020; 4(2): 1-11. doi: 10.1002/jbm4.10249
- Washio A, Kitamura C, Nishihara T. Possible Involvement of Smad Signaling Pathways in Induction of Odontoblastic Properties in KN-3 Cells by Bone Morphogenetic Protein-2: A Growth Factor to Induce Dentin Regeneration. Int J Dent. 2012; 2012(258469): 1-6. doi: 10.1155/2012/258469
- Kong Y, Hu X, Zhong Y, Xu K, Wu B, Zheng J. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Research & Therapy. 2019; 10: 378. 1-11. doi: 10.1186/s13287-019-1493-5
- Ding S, Zhang J, Tian Y, et al. Magnesium modification up-regulates the bioactivity of bone morphogenetic protein-2 upon calcium phosphate cement via enhanced BMP receptor recognition and Smad signaling pathway. Colloids Surf B Biointerface. 2016; 145(1): 140-151. doi: 10.1016/j.colsurfb.2016.04.045
- Zhang X, Chen Q, Mao X. Magnesium Enhances Osteogenesis of BMSCs by Tuning Osteoimmunomodulation. BioMed Research International. 2019; 2019(7908205): 1-25. doi: 10.1155/2019/7908205


