Review Article
Oral Submucous Fibrosis: A Clinical Review
1Associate Professor, Department of Oral & Maxillofacial Surgery, PMS College Of Dental Science & Research, Trivandrum, India
2Professor & Head, Department of Oral & Maxillofacial Surgery, PMS College Of Dental Science & Research, Trivandrum, India
3Professor, Department of Oral & Maxillofacial Surgery, PMS College Of Dental Science & Research, Trivandrum, India
4Junior Resident, Department of Oral & Maxillofacial Surgery, PMS College Of Dental Science & Research, Trivandrum, India
*Corresponding author: Sherin A Khalam, Associate Professor, Department of Oral & Maxillofacial Surgery, PMS College Of Dental Science & Research, Trivandrum, India, E-mail: drsherin666@gmail.com
Received: September 3, 2016 Accepted: September 9, 2016 Published: February 9, 2017
Citation: Khalam SA, Surej Kumar LK, Zacariah RK, Kurien NM, Raj P. Oral Submucous Fibrosis: A Clinical Review. Madridge J Dent Oral Surg. 2017; 2(1): 28-30. doi: 10.18689/mjdl-1000107
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Oral submucous fibrosis (OSMF) is a chronic disease affecting any part of the oral cavity. Epithelial atrophy, juxtaepithelial inflammation and fibrosis of the lamina propria are common findings. In this review we discuss various components of OSMF, including the classification, aetiology, clinical presentation, pathogenesis, and a brief overview of its management.
Keywords: Oral Submucous Fibrosis; Aetiology; Clinical Presentation; Pathogenesis; Management
Introduction
Oral submucous fibrosis (OSMF) is a chronic, insidious, disabling disease involving oral mucosa, the oropharynx, and, rarely, the larynx. It has been reported mainly in the Indian population, having been established in the Indian literature since the time of Sushruta [1]. The definition by the World Health Organization (WHO) of a precancerous oral condition: “a generalized pathological state of the oral mucosa associated with significantly increased risk of cancer” correlates well with the characteristics of OSMF [2-3].
Pindborg and Sirsat[3] gave a definition, as one of an insidious, chronic disease that affects any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by, or associated with, formation of vesicles, it is always associated with a juxtaepithelial inflammatory reaction followed by fibroelastic change of the lamina propria and epithelial atrophy that leads to stiffness of the oral mucosa and causes trismus and an inability to eat [1]. This is one of the most widely accepted definition of the disease.
In 1952, Schwartz proposed the term “atrophia idiopathies (tropica) mucosae oris” for this disease when he described the condition in 5 Indian women. The term “submucous fibrosis of the palate and pillars” were coined by Joshi [4]. Other terminologies suggested include “diffuse oral submucous fibrosis”, “idiopathic scleroderma of the mouth”, “idiopathic palatal fibrosis”, and “sclerosing stomatitis” [2-3]. Paymaster in 1956 [5] first described its premalignant nature. The term “submucous fibrosis” was used by Pindborg and Sirsat although they suggested that a more appropriate name would be “juxtaepithelial fibrosis” [1]. Ramnathan called it an Asian analog of sideropenic dysphagia, when he suggested that OSMF may be a mucosal change secondary to chronic iron deficiency [3].
Classification
Various classification and staging systems have been mentioned in literature [6]. Pindborg and Sirsat [1] in 1966 proposed one of the earliest classification based on histopathological features and was later updated by Utsunomiya et al [7] in 2005. The classification did not give any description of the epithelial component of the disease [6]. Wahi et al [8]. presented the first clinical classification based on symptoms, and since then several others proposed different classifications. Khanna and Andrade [9] have successfully combined histopathological and clinical features of the disease, and proposed a staging mainly to aid in surgical management.
Aetiology
The condition is thought to be multifactorial in origin; studies have shown that areca-nut is the main aetiological factor in OSMF [10]. Various auto antibodies and specific human leucocyte antigens (HLA) in some patients have indicated an autoimmune role as well as a genetic predisposition for the disease [11]. Other possible aetiological factors include hypersensitivity, capsaicin in chillies, malnutrion, iron, zinc, and deficiencies in essential vitamins [12-13].
Clinical presentation
The disease can be classified clinically into two phases: An eruptive phase and the fibrosis induction phase. These two phases appear in a cyclic manner. Initially, most patients present with a burning sensation or intolerance to spicy food, and they may have vesicles, particularly on the palate. Ulceration and dryness of the mouth is later followed by fibrosis of the oral mucosa, which leads to rigidity of the lips, tongue, and palate, and trismus [12]. Petechiae occur most often on the tongue followed by the labial and buccal mucosa [2,14]. useful clinical sign is pain on palpation in the sites where submucosal fibrotic bands are developing [14], (Figure 1) and trismus (Figure 2) is caused mostly by fibrosis in the dense tissue around the pterygomandibular raphae [14]. In advanced stages the patients may experience referred pain to ear,dysphagia [14-15] due to fibrosis involving nasopharynx or oesophagus and even,deafness due to fibrosis of Eustachian tube [15-16].
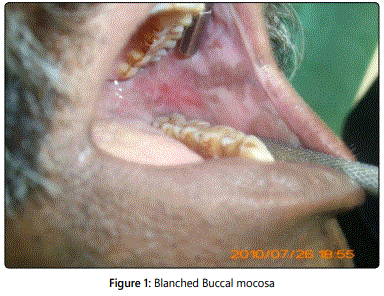
Palpable fibrous bands with blanched, opaque oral mucosa [10,13,14] is the most identifying clinical signs (Figure1) Furthermore, the overlying epithelium may become dysplastic and malignant. Restricted mouth opening interferes with examination of the oral mucosa, and makes early diagnosis of cancer a difficult task [10,15,17].

Pathogenesis
Although various factors and theories have been put forth to explain the pathogenesis of OSMF, nothing is fully conclusive. Studies have shown areca-nut to play a key primary role in the development of OSMF.
It contains alkaloids, flavonoids, and copper, which all interfere with homeostasis of the extracellular matrix. Four alkaloids:arecoline, arecaidine, guvacine, and guvacoline are known to stimulate fibroblasts to produce collagen [10]. Flavonoids like tannins and catechins inhibit collagenase, stabilise the collagen fibrils, and render them resistant to degradation by collagenase [10,13]. The localised mucosal inflammation caused results in the recruitment of activated T-cells and macrophages that lead to an increase in cytokines and tumour growth factor beta (TGF- β) [15]. The latter considerably increases the production of collagen by activating procollagen genes, and upregulating procollagen proteinase enzymes and lysyl oxidase activity [7]. Simultaneously, TGF-β inhibits collagen degradation by activating the tissue inhibitor of matrix metalloproteinase (TIMP) genes and plasminogen activator inhibitor (PAI) [15]. The high concentration of copper in areca-nut has been found to stimulate lysyl oxidase activity, an enzyme essential to the final cross-linking of collagen fibres [15]. Increased copper has been seen in mucosa affected by OSMF, which supports its role in fibrogenesis by enhancing lysyl oxidase activity [10]. Continually chewing areca-nut leads to increased activity of the masticatory muscles, depletion of glycogen, and muscle fatigue. The reduced blood supply following fibrosis further promotes muscle fatigue and causes extensive degeneration and fibrosis in the muscles [9].
Two other possible concurrent mechanisms to explain the pathogenesis of OSMF are autoimmune factors and genetic predisposition [13]. This has been substantiated by the presence of circulating immune complexes, immune globulins and auto antibodies in some patients with OSMF, as well as altered cellular and humoral responses [10,13]. Genetic susceptibility is supported by raised HLA-A10, -B7, and DR3 in OSMF patients compared with normal controls [14]. The familial occurrence of the disease has been reported from India and South Africa [10,13]. It seems likely that OSMF is a multifactorial disease with initiators, promoters, and other modifying cofactors. The loss of equilibrium of extracellular matrix and continuous deposition of extracellular matrix in OSMF is currently well accepted [13].
Management
The various treatment options for the management of OSMF can be grouped under following headings: surgical, physical, and medical treatments. Medical treatment includes dietary supplements such as vitamins and antioxidants, the use of anti-inflammatory drugs mainly corticosteroids, use of proteolytic agents such as hyaluronidase, anti cytokines have been given orally, topically or by submucosal injections [17] [18]. Physical treatment measures aimed to influence the remodelling of tissue by using movement include exercises and physiotherapy, various splints or other devices to improve mouth opening, or localised heat such as with microwave diathermy [19,20]. Kerr et al [18]. Recently hypothesised that cessation of the habit alone may have a considerable effect more on the symptoms of OSMF than on reversing fibrosis.
Surgical treatment, used mainly to manage trismus, involves incising and releasing the fibrotic areas, and leads to further scarring and fibrosis. The introduction of pedicled tissue, such as a buccal fat pad, nasolabial or platysmal flaps, or free tissue transfer in an attempt to release fibrosis [19,20] is one approach but results are variable. Physical treatment measures combined with other interventions are usually used for treating OSMF and surgery is reserved only for established cases.
Conclusion
OSMF is a debilitating but preventable disease and considering the malignant potential of the diseases, early diagnosis and proper management is essential in reducing the mortality of oral cancer.
References
- Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral MedOral Pathol. 1966; 22(6): 764–79.
- Rajendran R. Oral submucous fibrosis: etiology, pathogenesis, and future research. Bull World Health Organ. 1994; 72(6): 985–96.
- World Health, Organization. Guide to epidemiology and diagnosis oforal mucosal diseases and conditions. Community Dent Oral Epidemiol. 1980; 8: 1–26.
- Paymaster JC. Cancer of the buccal mucosa; a clinical study of 650 casesin Indian patients. Cancer. 1956; 9(3): 431–5. doi: 10.1002/1097-0142
- Ranganathan K, Mishra G. An overview of classification schemes fororal submucous fibrosis. J Oral Maxillofac Pathol. 2006; 10: 55–8.
- Utsunomiya H, Tilakaratne WM, Oshiro K, et al. Extracellular matrix remodeling in oral submucous fibrosis: its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med. 2005; 34(8): 498–507. doi: 10.1111/j.1600-0714.2005.00339.x
- Wahi PN, Luthra UK, Kapur VL. Submucous fibrosis of the oral cavity. Histomorphological studies. Br J Cancer. 1966; 20(4): 676–87.
- Khanna JN, Andrade NN. Oral submucous fibrosis: a new concept in surgical management. Report of 100 cases. Int J Oral Maxillofac Surg. 1995; 24(6): 433–9.
- Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral Oncol. 2006; 42(6): 561–8. doi: 10.1016/j.oraloncology.2005.08.005
- Warnakulasuriya KA, Trivedy C, Maher R, Johnson NW. Aetiology of oral submucous fibrosis. Oral Dis. 1997; 3: 286–7.
- Sinor PN, Gupta PC, Murti PR, et al. A case control study of oral sub mucous fibrosis with special reference to the etiologic role of areca nut. J Oral Pathol Med. 1990; 19(2): 94–8.
- Angadi PV, Rao SS. Areca nut in pathogenesis of oral submucous fibrosis:revisited. Oral Maxillofac Surg. 2011; 15(1): 1–9.11. doi: 10.1007/s10006-010-0219-8
- Rajendran R. Oral submucous fibrosis. J Oral Maxillofac Pathol. 2003; 7: 1–4.
- Aziz SR. Oral submucous fibrosis: case report and review of diagnosisand treatment. J Oral Maxillofac Surg 2008; 66(11): 2386–9. doi: http://dx.doi. org/10.1016/j.joms.2008.06.064
- Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluationof different treatment methods for oral submucous fibrosis. A 10-yearexperience with 150 cases. J Oral Pathol Med. 1995; 24(9): 402–6.
- Kerr AR, Warnakulasuriya S, Mighell AJ, et al. A systematic review of medical interventions for oral submucous fibrosis and future research opportunities. Oral Dis. 2011; 17(Suppl. 1): 42–57. doi: 10.1111/j.1601-0825.2011.01791.x
- Huang JJ, Wallace C, Lin JY, et al. Two small flaps from one antero lateral thigh donor site for bilateral buccal mucosa reconstruction after release of submucous fibrosis and/or contracture. J Plast Reconstr Aesthet Surg. 2010; 63(3): 440–5. doi: 10.1016/j.bjps.2008.12.010
- Wei FC, Chang YM, Kildal M, Tsang WS, Chen HC. Bilateral small radial forearm flaps for the reconstruction of buccal mucosa after surgical release of submucosal fibrosis: a new, reliable approach. Plast Reconstr Surg. 2001; 107(7): 1679–83.


