Research Article
Polymorphism of TNF-α as a Biomarker of Diabetic Nephropathy in patients of Telangana Region
1Department of Genetics, Bhagwan Mahavir Medical Research Centre, Masab Tank, Hyderabad-500004, Telangana, India
2Consultant Physician, Mahavir Hospital and Research Centre, Masab Tank, Hyderabad-500004, Telangana, India
*Corresponding author: Kaiser Jamil, Head of Genetics Department, Bhagwan Mahavir Medical Research Centre, 10-1-1, Mahavir Marg, Masab Tank, Hyderabad, Telangana, India, E-Mail: kj.bmmrc@gmail.com
Received: November 01, 2019 Accepted: November 12, 2019 Published: November 19, 2019
Citation: Jamil K, Haq O, Ahmed Z, Joshi S. Polymorphism of TNF-α as a biomarker of Diabetic Nephropathy in patients of Telangana Region. Madridge J Case Rep Stud. 2019; 4(1): 154-158. doi: 10.18689/mjcrs-1000141
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Diabetic Nephropathy (DN) is one of the complications in patients with prolonged diabetes. Among the genetic risk factors for DN, TNF-α, an anti-inflammatory cytokine is proposed to act in a paracrine/autocrine manner and is hypothesized to be associated with insulin resistance. In the current study, the relationship of the G→C variant of the TNF-α gene in patients, associated with other biochemical parameters with DN was investigated.
Methods: Demographic factors of the study group were obtained by directly interviewing the study group. Biochemical and diagnostic parameters of the study subjects, plasma glucose levels (fasting and postprandial), renal function tests (Urea, creatinine) were obtained from the patientʼs records. Genomic DNA was extracted from peripheral blood samples of 50 type II Diabetes Mellitus (T2DM) and 50 non-diabetic control subjects. The TNF-α (G→C) polymorphism was analysed using polymerase chain reaction (PCR), followed by restriction fragment length (RFLP) polymorphism analysis.
Results: Using statistical analysis we could correlate demographic parameters with genotyping results, and we found that 50% patients were GG homozygotes (wild type), 30% were GC heterozygotes, and 20% were CC homozygotes. This suggests that lowgrade inflammation could be one of the determinants in the pathogenesis of insulin resistance and T2DM.
Conclusion: We conclude from this preliminary study that TNF-α G→C genotypes may be useful biomarker for the early diagnosis of T2DM patients with insulin resistance and nephropathy.
Keywords: Diabetes mellitus; TNF-α; Snps; Diabetic nephropathy; Chronic kidney disease; End stage renal disease; Biomarkers; Insulin resistance.
Introduction
The International Diabetes Federation (IDF) estimates the total number of people in India with diabetes to be around 50.8 million in 2010, rising to 87.0 million by 2030. Diabetes mellitus (DM) commonly referred as diabetes is a metabolic disorder in which the blood sugar levels are elevated because the body does not produce enough insulin that can metabolise the blood sugar [1,2]. However there could be other reasons for diabetic conditions, such as insulin resistance or autoimmunity. Years of poorly managed DM can lead to multiple medical irregularities that can affect small vessels (microvascular), large vessels (macrovascular) or both [3] including nephropathy, retinopathy, neuropathy, angina pectoris, and peripheral arterial disease. Nephropathy disorder is most commonly observed in patients with prolonged diabetes even though the other microvascular diseases due to diabetes are also observed. Although the commonness of nephropathy eaps up with chronic diabetes, it levels out at ~15 years after diagnosis [4].
Diabetic nephropathy is due to longstanding diabetes mellitus, and is a prime reason for patients to undergo dialysis in many developed countries. It is classified as a small blood vessel (microvascular) complication of diabetes. Diabetic nephropathy (or diabetic kidney disease is a progressive kidney disease caused by damage to the capillaries in the kidneysʼ glomeruli. It is characterized by nephrotic syndrome and diffuses across the glomeruli. Diabetic nephropathy occurs in both, type 1 (formerly called insulin-dependent or juvenile onset) and type 2 (formerly called non-insulindependent or adult onset) diabetes mellitus.
A majority of the cases of diabetic nephropathy presents with proteinuria, which progressively gets worse as the disease progresses, and is almost uniformly associated with hypertension. It is also one of the most significant long-term complications in terms of morbidity and mortality for individual patients with diabetes. Clinically, diabetic nephropathy is expected to become the most frequent cause of End Stage Renal Disease (ESRD) [5,6]. Progressive increase in proteinuria and decline in Glomerular Filtration Rate (GFR), hypertension, risk of high cardiovascular morbidity and mortality are the clinical characteristics of Diabetic Nephropathy [7]. Early diagnosis and early treatment using nephroprotective therapy have the potential to prevent the progression of Diabetic Kidney Disease (DKD) towards end stage renal disease and to improve patientʼs prognosis [8-10].
Cytokines are signalling molecules that mediate endocrine, paracrine and autocrine signalling functions and regulate the maturation, growth and responsiveness of cell populations. They are important in the host responses to infection, inflammation, trauma, cancer, reproduction etc, and play an important role in health and disease. Indeed, greater amounts of TNF-α are found to be induced from the glomerular basement membranes and renal cells [11,12]. Higher levels of TNF-α are found in the urine and serum of DN patients than in diabetes patients with no nephropathy, which is mainly produced by monocytes, macrophages and T-cells and is a pleotropic inflammatory cytokine. Other inflammatory mediators are the activators of second messenger system, transcription factors, synthesis of other cytokines, growth factors, receptors, cell adhesion molecules and also enzymes involved in the synthesis of other inflammatory mediators [11,13]. A variety of effects on different renal structure are induced by TNF-α, which may act in a paracrine or autocrine manner and ironically is capable of getting synthesized by renal cells. Moreover TNF-α has a direct effect on protein permeability barrier of the glomeruli independent of the effects of recruited inflammatory cells. These findings suggest that the inflammatory cytokines may participate in the pathogenesis of DN and can be predictive biomarkers. Hence, such studies, that show a strong association between cytokine gene promoter polymorphisms and modulation of transcript levels with susceptibility to nephropathy in diabetes subjects [14,15] was the subject of our investigation.
Recent studies show that there are elevated levels of inflammatory molecules in the plasma of diabetic patients and the concentration of these molecules increases as diabetic condition progresses[16,17]. The accumulation of inflammatory cell in the kidney is closely associated with DN[18,19]. Inhibition of recruitment of inflammatory cell into the kidney has been shown to be protective in some experimental DN[20]. However, not much information is available on effective measures to prevent the progression of DN. Even though there are strategies based on stringent control of modifiable risks like hypertension, dyslipidaemia and glucose levels; the ultimate progression of DN cannot always be prevented. Therefore, the identification of therapies through the gene involved in the progression of DN will help us control the risk factors [21].
Uncovering the genetic changes in diabetes may be important in defining the functional role of specific genetic alterations for developing potential biomarkers. Genetic causes and mechanisms of type 2 diabetes is largely unknown. However, it is understood that single nucleotide polymorphisms (SNP) in identified genes are one of many mechanisms that lead to increased risk for type 2 diabetes. Since diabetic nephropathy is the most common complication of DM and is categorized as the end stage disease, we tried to focus on this group with an aim to investigate the inflammatory marker that is involved in the pathogenesis of diabetic nephropathy. This helps in identifying the genetic variants that correlates with phenotypic abnormalities. Further the objectives of the study included, determination of the risk factors that include physical activities, BMI, obesity and demographics of the patients in order to understand their aetiology.
Information on genetic causes and mechanisms of type 2 diabetes is lacking. Hence our aim was to investigate the inflammatory markers that are involved in the pathogenesis of diabetic nephropathy and could serve as predictive or diagnostic biomarker. These markers can lead us to the drug discovery process, to save thousands of DN patients.
Materials and Methods
Patients and ethics committee approval
This case control study was conducted from July 2017 till July 2018 at Bhagwan Mahavir Medical Research Centre, Hyderabad, India, which is a tertiary public hospital. Ethics Committee approval was obtained from the Hospitalʼs review board following Helsinkiʼs declaration. Signed informed consents were obtained from patients before collecting data and blood samples (1-2 ml/subject). Controls comprised of healthy, non-smoking and non-alcoholic subjects with no past/current medical history of any serious health condition. Patients included dialysis subjects who attended the Dialysis centre at the hospital. Demographic parameters were collected in a structured questionnaire and lipid profile and other biochemical parameters were obtained from patientsʼ records. Diabetic nephropathy was characterized by the following criteria: persistent albuminuria (>300 mg/d or >200 μg/min) that is confirmed on at least 2 occasions 6-12 months apart, progressive decline in the glomerular filtration rate (GFR), and elevated arterial blood pressure.
Genomic studies
DNA was extracted from whole blood samples collected from the patients in EDTA vacutainers, through the salting out procedure [22] and checked for its purity by nanodrop method. The extracted DNA was preserved in TE buffer at -20°C till further use. A polymerase chain reaction (PCR) was performed using the primers of TNF--α: forward-5ʼAGG CAA TAG GTT TTG AGG GCC AT-3ʼ and reverse-5ʼGAG CGT CTG GCTG GG TG-3ʼ. The PCR amplification included denaturation at 94°C for 12 mins and 94°C for 30 sec followed by annealing at 59°C for 1 min, extension at 72°C for 2 mins, 35 cycles of amplification and final extension at 72°C for 10 mins. A Restriction Fragment Length Polymorphism (RFLP) procedure was conducted on the amplified products to identify SNPs using NocI restriction enzyme. The RFLP mix contained 0.5 μl of enzyme, 2 μl of buffer, 18 μl of PCR product, and 10 μl of water. The samples were first incubated at 37°C for 16 hrs for enzyme digestion, then the enzyme was deactivated by further incubation at 65°C for 20 min. The digested fragments were visualized and identified on 3% gel.
Demographic and biochemical parameters
Patients demographic parameters were collected in a structured questionnaire and lipid profile and other biochemical parameters were obtained from patientsʼ records which were useful for co-relational studies and for inclusion or exclusion criteria.
Results
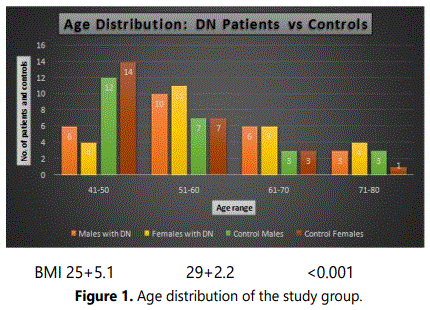
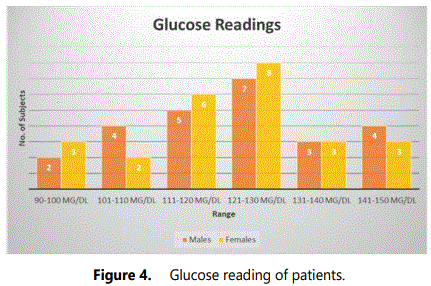
A total of 100 subjects consisting of 50 patients and 50 controls were included in the study. There was equal distribution of male and female subjects in each group (viz., 25 males and 25 females in each group). About 6 male and 4 female DN patients were in age range of 41-50, 10 males and 11 females were in age range 51-60, 6 males and 6 females in age range 61-70 and 3 males and 4 females in age range 71-80 as depicted in figure 1. Approximately 22 males and 21 females had type 2 DM and 3 males and 4 females had type 1 DM. The commonly observed symptoms among the patients along with albumin and serum readings are presented in figures 2 and 3, respectively. The most common symptom in both the genders was dry mouth (viz., 18 males and 17 females). Symptoms like vomiting, swollen limbs were observed only in females and intense hunger in males from the study group. Other symptoms observed are decreased appetite in 8 males and 8 females, night sweats in 3 males and 3 females, frequent urination in 15 females and 5 males, increased thirst in 10 males and 5 females, weakness in 10 males and 3 females from the study group. Albumin readings were high in most of the patients and serum creatinine was under and above the reference range among the studied subjects. The variations in glucose and blood urea readings of the patients are presented in figures 4 and 5. Most of the patients have glucose readings higher than the normal. Blood urea readings were normal in most of the patients except a few that includes 12 female and 9 males whose blood urea readings were more than the normal as depicted in the mentioned figure. Most of the patients (80%) in the hospital were not physically active and these patients had much longer inpatient stay when compared to the subject patients who were involved in regular physical exercise.
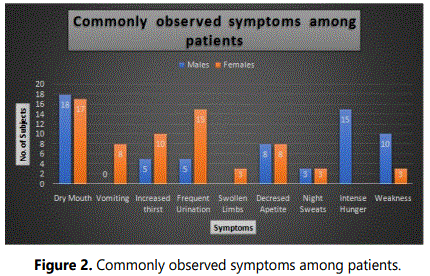
The figure 2 shows that one individual has 2-3 commonly observed symptoms. Hence the numbers presented in the above graph indicates the multiple kinds of symptoms which an individual can have.
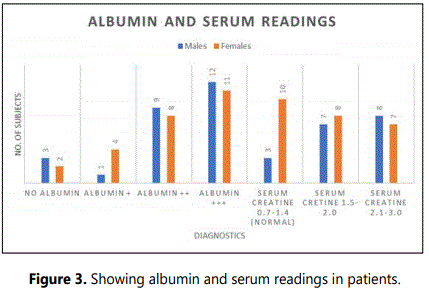
Normal reference range of diagnostic parameters
ALBUMIN=0-8 mg/dl (+ indicates High Albumin, ++ indicates Higher Albumin and +++ indicates much Higher Albumin levels than the normal range)
- SERUM CREATINE=0.7-1.4 mg/dl
- GLUCOSE=70-110 mg/dl
- BLOOD PRESSURE=120/80 mmHg.
- BLOOD UREA=15-45 mg/dl
The Blood pressure readings among all the subjects were in normal range while other diagnostic parameters varied.
Abstract
Almost all the patients regardless of gender had high albumin and creatinine in their urine and serum respectively.
Discussion
In 2014, Gheith et al. had highlighted the prevalence of DN in different countries [23]. Caucasian patients with type 1 DM were more progressive to kidney disease than the patients with type 2 DM. In Pima Indians the occurrence of DN was very interesting. It was reported that around 50% of Pima Indians with type 2 DM developed nephropathy after 20 years of the disease, and 15% of them were already in the terminal stages of kidney failure. In late 1990s the occurrence of DN in patients beginning kidney replacement doubled in the United States but the trend had been decreasing due to better prevention and treatment in the earlier diagnosis stages. Approximately 44% of the new patients entering dialysis in the United States were diabetics. Epidemiological differences were recorded among European countries, mainly Germany. Renal replacement proportion therapy was higher in these countries than what was reported in the United States. In Heidelberg, nearly 60% of the patients admitted for renal replacement were diabetics in 1995. Even in the countries that are known to have a lower incidence of type 2 DM such as Denmark and Australia there was an increase in ESKD. DN affects males and females equally, the role of the age in the development of the disease is still unclear although the mean age of the patients who reaches ESKD is about 60 years and the DKD was higher among elderly persons who have type 2 diabetes for a longer time. In Pima Indians earlier the onset of diabetes, the higher is the risk of progression of ESKD. The severity incidence of DN was 3 to 6-fold higher in blacks than in whites. Similarly, the commonness of DN was more in Mexican Americans and Pima Indians. This suggests that the socioeconomic factors such as diet, poor control of hyperglycaemia, hypertension and obesity have a primary role in the development of DN. The prevalence of any type of CKD was more in the people of Asian origin as reported in some studies. Six Arabic-speaking countries like Kuwait, Lebanon, Qatar, Saudi-Arabia, Bahrain and the United Arab Emirates (U.A.E), were among the top countries in terms of the prevalence of type 2 DM. Cross sectional study in Egypt had shown that 42% of the diabetics had nephropathy. In Jordan 33% of the diabetics at the national diabetes centre had nephropathy and in Libya, 25% of the patients had nephropathy.
King GL showed in his article [13], that the inflammatory processes were implicated in the onset of diabetes and the progression of the complication. Through the activation of NF-κB pathway the development of insulin resistance in type 2 diabetes was found to be associated with the release of inflammatory cytokines and other mediators from adipose cells. Obesity, a high fat diet, hyperglycaemia, PKC activation and oxidative stress were the other factors that activate the NF-κB pathway with the development of insulin resistance. Hyperglycaemia is the major risk factor for the development of micro-vascular diabetic irregularities such as retinopathy, neuropathy and nephropathy.
Hasegawa et al. [24] hypothesized the pathogenic role of TNF-α in the progression of diabetic nephropathy. In this regard many mechanisms have been proposed by which TNF-α may induce renal injury directly [25]. Stimulation of production of endothelin-1 may be carried out by TNF-α that leads to dysregulation of vascular tone that result in reduced intra-renal flow of blood and glomerular filtration rate. This mechanism may also disrupt endothelial intercellular junctions affecting the integrity of glomerular filtration barrier that causes an increase in its permeability and albuminuria. TNF-α may also have cytotoxic direct effects on glomerular podocytes and tubular cells inducing apoptosis and cell death. This might also alternatively cause the activation of protein kinase/phospatidylinositol-3 kinase pathway or activation of NADPH oxidase culminating into the generation of ROS and consequential cellular damage. With regards to the glomerular podocytes, it may reduce the expression of nephrin via activation of PI3K/Akt pathway and reduces Act activity in diabetes resulting in reduced cell survival. These effect of TNF-α support its role as an inducer as well as driver of renal injury by regulating the expression of other downstream cytokines.
Despite of huge literature about the pathogenetic role of inflammatory cytokines in the pathogenesis of DN, it was needed to determine whether TNF-α was suitable target or biomarker for therapeutic intervention to control the progression of DN. Relationship between elevated serum and urinary levels of TNF-α have been reported in many studies along with abnormal urinary protein excretion and reduces GFR. In patients with diabetic nephropathy and murine models of experimental diabetes, the expression of TNF-α was found to be increased. Furthermore, higher levels of circulating TNF-α concentration was associated with the loss of renal function [26].
Therefore, studies on the factors that are involved in the progression of the disease is vital as it helps us in finding out the targets that could help us prevent the development of disease. Diabetic nephropathy is considered as inflammatory disease and several reports have demonstrated the association of diabetic nephropathy with inflammasome activation [27].
The control of inflammatory processes may be useful in the therapy of diabetic nephropathy. As there is limited experience available for the inhibition of inflammatory cytokines in DN; it is beneficial collecting and mounting evidence for the properties of inflammatory genes.
Our research involved the study of TNF-α which is one of the inflammatory cytokines. This is the first study that is done in Indian population so far and the results observed are vital and mounts evidence about the involvement of this inflammatory gene in the progression of DN.
Conclusion
These findings suggest that inflammatory cytokines may participate in the pathogenesis of diabetic nephropathy, TNF-α the inflammatory markers showed an increased level in all DN patients irrespective of gender hence can be proposed as a biomarker of DN.
Acknowledgements
We are thankful to Dr. Asma Sultana for reviewing the manuscript and to Prashant Ch. and Vanitha Research scholars for their help, and the Research Director BMMRC for his encouragement, and we are thankful to all the patients who took part in this study voluntarily.
Conflict of Interest
The authors declare no conflict of interest.
References
- World Health Organization. About diabetes. 2014.
- Brutsaert EF. Diabetes Mellitus (DM) and Disorders of Blood Sugar Metabolism. MSD Manuals Professional Version. 2019.
- Brutsaert EF. Diabetes mellitus and disorders of carbohydrate metabolism. MSD Manuals Professional Version. 2019.
- Amod A, Ascott-Evans BH, Berg GI, et al. The 2012 SEMDSA Guideline for the Management of Type 2 Diabetes (Revised). Guideline Committee. JEMDSA. 2012; 17(2): S1-S95.
- Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis. 2018; 71(6): 884-895. doi: 10.1053/j.ajkd.2017.10.026
- Butt S, Hall P, Nurko S. Diabetic nephropathy. Cleveland Clinic. 2010; 1-14.
- Żyłka A, Dumnicka P, Kuśnierz-Cabala B, et al. Markers of glomerular and tubular damage in the early stage of kidney disease in type 2 diabetic patients. Mediators Inflamm. 2018; 7659243. doi: 10.1155/2018/7659243
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017; 12(12): 2032-2045. doi: 10.2215/CJN.11491116
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation Practice guidelines for chronic kidney disease; evaluation, classification, and stratification. Ann Intern Med. 2003; 139(7): 605. doi: 10.7326/0003-4819-139-2-200307150-00013
- Hasegawa G, Nakano K, Kondo M. Role of TNF and IL-1 in the development of diabetic nephropathy. Nefrologia. 1995; 15(1): 1-4.
- Kinaan M, Yau H, Martinez SQ, Kar P. Concepts in Diabetic Nephropathy: From Pathophysiology to Treatment. Journal of Renal and Hepatic Disorders. 2017; 1(2): 10-24. doi: 10.15586/jrenhep.2017.17
- King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008; 79: 1527-1534. doi: 10.1902/jop.2008.080246
- Hameed I, Masoodi SR, Malik PA, Mir SA, Ghazanfar K, Ganai BA. Genetic variations in key inflammatory cytokines exacerbates the risk of diabetic nephropathy by influencing the gene expression. Gene. 2018; 661: 51-59. doi: 10.1016/j.gene.2018.03.095
- Fathy SA, Mohamed MR, Ali MAM, EL-Helaly AE, Alattar AT. Influence of IL-6, IL-10, IFN-γ and TNF-α genetic variants on susceptibility to diabetic kidney disease in type 2 diabetes mellitus patients. Biomarkers. 2019; 24(1): 43-55. doi: 10.1080/1354750X.2018.1501761
- Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor α and blood cytokine production in type 2 diabetes. Life Sci. 2000; 67(3): 291-300. doi: 10.1016/s0024-3205(00)00622-6
- Bruno G, Merletti F, Biggeri A, et al. Progression to overt nephropathy in type 2 diabetes: the Casale Monferrato study. Diabetes Care. 2003; 26(7): 2150-2155. doi: 10.2337/diacare.26.7.2150
- Ferenbach D, Kluth DC, Hughes J. Inflammatory cells in renal injury and repair. Semin Nephrol. 2007; 27(3): 250-259. doi: 10.1016/j.semnephrol.2007.02.001
- Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004; 65(1): 116-128. doi: 10.1111/j.1523-1755.2004.00367. x
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005; 16(6): 1711-1722. doi: 10.1681/ASN.2004070612
- Williams ME, Tuttle KR. The next generation of diabetic nephropathy therapies: an update. Adv Chronic Kidney Dis. 2005; 12(2): 212-222. doi: 10.1053/j.ackd.2005.01.011
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988; 16(3): 1215. doi: 10.1093/nar/16.3.1215
- Gheith O, Farouk N, Nampoory N, HalimM, Al-Otaibi T. Prevalance of Diabetic Nephropathy. Diabetologia Croatica. 2014; 43(2): 35-46.
- Hasegawa G, Nakano K, Sawada M, et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 1991; 40(6): 1007-1012. doi: 10.1038/ki.1991.308
- Sun L, Kanwar YS. Relevance of TNF-α in the context of other inflammatory cytokines in the progression of diabetic nephropathy. Kidney Int. 2015; 88(4): 662-665. doi: 10.1038/ki.2015.250
- Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015; 87(2): 281-296. doi: 10.1038/ki.2014.285
- Shahzad K, Bock F, Dong W, et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015; 87(1): 74-84. doi: 10.1038/ki.2014.271




