Case Report
Giant Primary Amelanotic Scalp Melanoma: Report of a Rare Case and Review of the Literature
1Department of Dermatology and Venereology, Hassan II University Hospital, Fès, Morocco
2Department of Anatomic Pathology, Hassan II University Hospital, Fès, Morocco
3Department of Plastic Surgery, Hassan II University Hospital, Fès, Morocco
*Corresponding author: Safae Zinoune, Doctor, Department of Dermatology and Venereology, Hassan II University Hospital, Fès Morocco, Email: dr.zinounesafae@gmail.com
Received: March 6, 2019 Accepted March 12, 2019 Published: March 18, 2019
Citation: Zinoune S, Elloudi S, Saadani Hassani C, et al. Giant Primary Amelanotic Scalp Melanoma: Report of a Rare Case and Review of the Literature Madridge J Case Rep Stud. 2019; 3(1): 114-117. doi: 10.18689/mjcrs-1000128
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Giant cutaneous malignant melanoma is rarely observed in clinical practice. It has rarely been described in the literature. Melanoma of the scalp with amelanotic character is even rarer, it represents an aggressive subcategory with high rates of in-transit disease and poor disease-related and survival outcomes. In this paper, we document our experience with an elderly patient with a giant amelanotic melanoma of the scalp and review the literature.
Keywords: Melanoma; Amelanotic; Scalp; Dermoscopy; Skin Transplantation.
Introduction
Giant melanoma has been rarely described in the literature [1]. The term ‘giant melanoma’, although not formally defined, has been previously described as a large diameter melanoma, often over 10 cm [2]. The term ‘thick melanoma’ refers to a large malignant melanoma with a Breslow thickness of over 4 mm [3].
Melanomaʼs anatomic location has an important influence on tumor characteristics and prognosis. Scalp melanoma has a worse prognosis than melanoma elsewhere, though the reasons for this are poorly understood [4]. Cutaneous head and neck melanoma (CHNM) described as having clinical and histological features that are different from melanomas in other locations [3,5]. Scalp melanomas comprise 7% of all cutaneous melanomas and have higher recurrence and mortality rates than other CHNM [6].
Our case demonstrates a remarkable outcome from surgical excision alone with no loco regional or distant metastatic spread over 4 years from presentation.
Case Report
A 79-year-old man was referred to our department of dermatology following a 1-year history of an asymptomatic progressively enlarging mass on his scalp. He had a medical history of prostate cancer treated 5 years ago and a euthyroid multinodular Goiter.
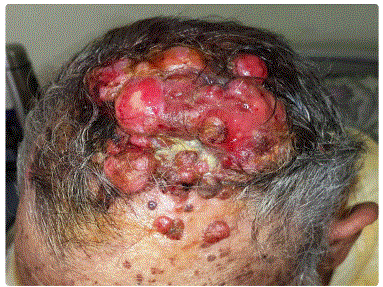
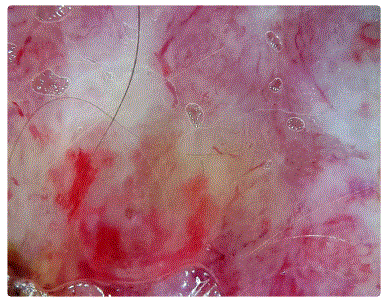
On clinical examination, there was a reddish, firm, bleeding andmultinodular tumor located on the scalp with purulent exudation. The dimensions of the tumor were 14 cm × 11 cm × 3 cm (Figure 1). We also noticed the presence of multiple depigmented, erythematous macules with irregular borders, on the trunk. Dermoscopy of the tumor showed polymorphous vascularization with milky red and amorphous white areas (Figure 2). Clinically, he had no palpable cervical lymphadenopathy and no other significant abnormalities at the somatic examination.
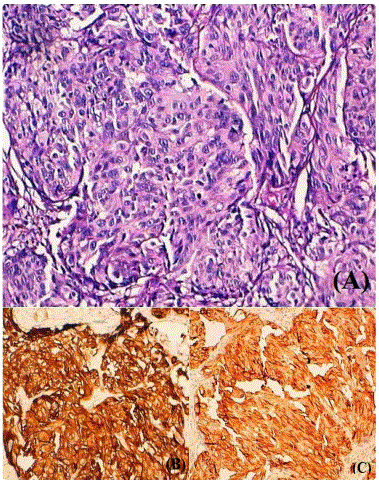
Biopsy specimen was consistent with nodular invasive malignant melanoma, Clark level IV, Breslowʼs thickness of at least 7 mm, the tumour was strongly positive for HMB45 and Melan-A confirming the diagnosis of malignant melanoma (Figure 3). Computed tomographic scan of head showed no obvious cranial involvement by the lesion. A full workup for metastatic disease was done, including ultrasound of cervical ganglionic areas, computed tomography of the thorax, abdomen, and pelvis, which did not show any suspicious lesions, Serum lactate dehydrogenase (LDH) level was normal.
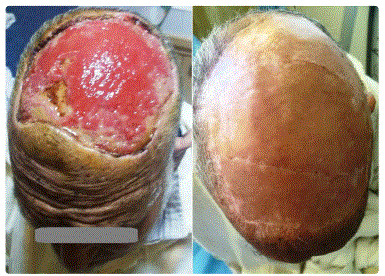
The patient was preoperatively staged as T4N0M0. Wide local excision with 3 cm margins was performed. Then, after the anatomopathological control of margins, the defect was covered with a split-thickness skin graft taken from the left thigh, with good evolutionà (Figure 4). This patient is still alive over 4 years later with no locoregional or distant metastatic spread.
Discussion
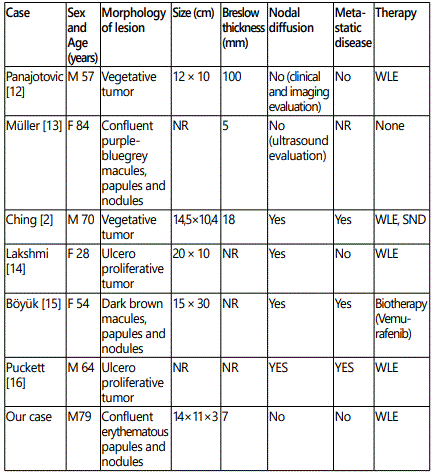
There are very few reports on giant melanoma in literature, because patients usually come for treatment earlier, and practitioners are well aware of the catastrophic consequences of neglecting such tumors [1]. A systematic literature review, limited to the English language, has shown only 6 patients with scalp melanoma appearing as a giant cutaneous tumor (Table 1). The dimensions of melanoma in our case make it one of the rarest.
Primary melanoma of the scalp represents a unique sub-category with an aggressive phenotype characterized by high rates of local, in-transit metastases, and regional recurrence as well as poor disease-free survival outcomes [7]. Itʼs more likely to be rapidly growing, a melanotic and nodular in subtype. They tend to occur in older men and, by inference; it is likely that male pattern alopecia and chronic solar damage play a role in their causation. High-risk histological features such as increased Breslow thickness, nodular subtype, and satellite metastases may explain the poor prognosis of scalp melanoma [4]. The reason for local or loco-regional spread in some massive melanomas without distant metastases is unknown, although some authors have suggested that it could be ascribed to the intrinsic biological behavior of the tumor and patientʼs immunological response to the melanoma cells [1]. The presence of depigmented lesions vitiligo-like in our patient testifies the activation of its immune system which may explain the absence of locoregional and distant metastatic spread.
Amelanotic or hypomelanotic melanomas are more common in chronically sun-exposed areas. There increased relative incidence at other sites such as the scalp, particularly in men, may be related to the frequency of certain histologic subtypes such as nodular and desmoplastic melanoma. They are considered as forms of poor prognosis [8]. Dermoscopy may be useful in the diagnosis of a melanotic melanoma by visualization of features associated with deep tumour extension (blue-whitish veil, polymorphous vessels, little blue-black colour, pseudolacunas) not visible to the naked eye [9]. In our case, the presence of polymorphous vessels and milky red areas on dermoscopy as well as the presence of depigmented lesions on clinical examination allowed to evoke the diagnosis of melanoma.
Regarding therapy of giant melanoma, there is no consensus on how best to manage this tumor given the heterogeneity of the limited cases described in the literature. In the currently available guidelines, the maximum recommended resection margin is 3 cm [10]. However, a recent study demonstrates that within the head and neck, a wider resection margin greater than 2 cm does not confer any additional survival benefit compared with a narrower margin [4]. Furthermore, the use of wider margins did not confer a survival benefit to patients with more advanced disease, such as sentinel lymph node spread. This is of particular importance in the head and neck region, where the use of smaller margins allows for the preservation of aesthetics and facial/sensory function [4].
Although giant scalp melanomas are treated with surgery, their size can pose significant difficulties in scalp reconstruction, especially if they require deep excision and the patient has comorbid medical conditions. For larger scalp wounds where primary wound closure is not possible, reconstructive technique include local flaps, tissue expanders, and skin grafting. Although skin grafts are an easy and reliable method, an adequate nutrient blood supply is needed for the graft to become incorporated. This may require an additional pericranial or subgaleal fascia flap or drilling into the diploe to allow formation of granulation tissue [11-16].
Conclusion
This case appears to be one of the rare cases in the literature in terms of its lack of successful management with no locoregional or metastatic spread in over 4 years. Giant melanoma of the scalp represents a surgical challenge due to their destructive nature. Even though they have significant metastatic potential, a subset of these tumors could have a more indolent clinical course. Genetic analysis may prove a valuable clinical tool to predict phenotype and to provide more individualized treatment options.
Conflict of Interest: No conflicts of interest.
References
- Di Meo N, Stinco G, Gatti A, et al. Giant melanoma of the abdomen: case report and revision of the published cases. Dermatol Online J. 2014; 20 (7).
- Thörn M, Adami HO, Ringborg U, Bergström R, Krusemo U. The association between anatomic site and survival in malignant melanoma. An analysis of 12,353 cases from the Swedish Cancer Registry. Eur J Cancer Clin Oncol. 1989; 25(3): 483-491.
- Ching JA, Gould L. Giant scalp melanoma: a case report and review of the literature. Eplasty. 2012; 12: e51.
- Xie C, Pan Y, McLean C, Mar V, Wolfe R, Kelly JW. Scalp melanoma: Distinctive high risk clinical and histological features. Australas J Dermatol. 2017; 58(3): 181-188.
- Han AY, Dhanjani S, Pettijohn K, Patel PB, John MAS. Optimal Resection Margin for Head and Neck Cutaneous Melanoma. Laryngoscope. 2018. doi: 10.1002/lary.27465
- Ozao-Choy J, Nelson DW, Hiles J, et al. The Prognostic Importance of Scalp Location in Primary Head and Neck Melanoma. J Surg Oncol. 2017; 116(3): 337-343. doi: 10.1002/jso.24679
- Sparks DS, Read T, Lonne M, et al. Primary cutaneous melanoma of the scalp: Patterns of recurrence. J Surg Oncol. 2017; 115(4): 449-454. doi: 10.1002/jso.24535
- Wee E. Clinically amelanotic or hypomelanotic melanoma: Anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018; 79(4): 645-651.e4. doi: 10.1016/j.jaad.2018.04.045
- Pizzichetta MA, Kittler H, Stanganelli I, et al. Dermoscopic diagnosis of amelanotic/hypomelanotic melanoma. Br J Dermatol. 2017; 177(2): 538-540. doi: 10.1111/bjd.15093
- Ahmed OA, Kelly C. Head and neck melanoma (excluding ocular melanoma): United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016; 130(S2): S133-S141. doi: 10.1017/S0022215116000852
- Desai SC, Sand JP, Sharon JD, Branham G, Nussenbaum B. Scalp reconstruction: an algorithmic approach and systematic review. JAMA Facial Plast Surg. 2015; 17: 56-66. doi: 10.1001/jamafacial.2014.889
- Panajotovic L, Dordevic B, Pavlovic MD. A giant primary cutaneous melanoma of the scalp-can it be that big? J Eur Acad Dermatol Venereol. 2007; 21(10): 1417-1418. doi: 10.1111/j.1468-3083.2007.02218.x
- Müller CS, Hinterberger L, Vogt T, Pföhler C. Giant melanoma of the scalp--discussion of a rare clinical presentation. BMJ Case Rep. 2011; doi: 10.1136/bcr.12.2010.3643
- Bhagya Lakshmi A, Sudhakar PV, Shamili M, Subhash R. Massive nodular melanoma scalp: a case report. Int J Res Med Sci. 2015; 3(4): 1002-1005.
- Böyük E, Bulur I, Saraçoğlu ZN, Erdem Ö. Giant Scalp Melanoma. Osmangazi Journal of Medicine. 2016; 38(3): 81-82.
- Puckett Y, Bui E, Dissanaike S. Management of skin defect following resection of Stage IV scalp melanoma: A case report. Int J Surg Case Rep. 2016; 29: 8-10. doi: 10.1016/j.ijscr.2016.10.027




