Research Article
Pyocyanin: A Powerful Inhibitor Of Bacterial Growth and Biofilm Formation
Department of Natural Sciences, School of Arts and Sciences, Lebanese American University, Beirut, Lebanon
*Corresponding author: Tarek Nawas, Department of Natural Sciences, School of Arts and Sciences, Lebanese American University, Beirut, Lebanon, Tel: +961 03 374619, E-mail: tnawas@lau.edu.lb
Received: December 6, 2018 Accepted: December 12, 2018 Published: December 18, 2018
Citation: Raji El Feghali PA, Nawas T. Pyocyanin: A Powerful Inhibitor of Bacterial Growth and Biofilm Formation. Madridge J Case Rep Stud. 2018; 3(1): 101-107. doi: 10.18689/mjcrs-1000125
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Pyocyanin, a pigment naturally produced by most Pseudomonas aeruginosa strains and noted to have a role in the well being of the organism and its pathogenicity, was studied for its role as an inhibitor of bacterial growth and biofilm formation of many clinical bacterial isolates. The organisms included in the study were 33 isolates of 11 different bacterial species. The results showed that most of the Gram-positive isolates from the 3 species included in the study: Staphylococcus aureus, Staphylococcus saprophyticus and Enterococcus fecal is were inhibited at low concentrations of pyocyanin. The results varied for the Gram-negative organisms tested. Whereas the growth of the Escherichia coli, Citrobacter koseri, Enterobacter cloacae and A. baumannii isolates was clearly inhibited even by low concentrations of pyocyanin, the growth of Klebsiella pneumonia, Proteus mirabilis and Morganella morganii was not affected even by higher concentrations of pyocyanin. The methanol extract of the pigment was highly effective in preventing the biofilm forming ability of all S. saprophyticus, A. baumannii, E. cloacae and K. pneumoniae isolates, 3 of the 4 P. mirabilis isolates and 2 of the 5 E. coli isolates. All the other isolates tested, also showed a moderate antibiofilm activity This study revealed that pyocyanin has a powerful inhibitory effect on the bacterial growth and/or biofilm forming ability of the numerous clinically significant isolates tested, a characteristic that can prove valuable in developing new drugs for the treatment and prevention of different bacterial infections, once its safety for regular use has been assessed.
Keywords: Pyocyanin; Antibacterial activity; Inhibition of biofilm formation; Bacterial pigment; Pseudomonas aeruginosa.
Introduction
Pseudomonas aeruginosa is a proteobacterial Gram-negative bacillus placed in the family Pseudomonadaceae based on the analysis of conserved macromolecules like the 16S ribosomal RNA [1]. Its metabolism relies mainly on aerobic respiration. It is motile and almost all of its strains are motile by the single polar flagellum that they possess [2,3]. P. aeruginosa can colonize many natural and artificial areas including soil, water, etc [4,5]. This is due to its ability to live not only in atmospheric conditions, but also in hypoxic atmospheres. Special features, including the different colors and pigments it produces, make P. aeruginosa easily recognizable. These pigments include pyocyanin (blue-green), pyoverdin (yellow, green and fluorescent), pyomelanin (light brown) and pyorubrin (red-brown) [6,7]. Pyocyanin, or the ‘blue pus’ (from pyocyneus), is produced by around 90-95 % of all P. aeruginosa isolates (Fordos, 1863; Gessard, 1984; Ran et al., 2003) [8,9,10]. Pyocyanin production is favored in media where iron is not present in high amounts, but it plays a major role in iron metabolism. Its mechanism of action relies mainly on its ability to reduce and release the iron from transferrin; this is important since P. aeruginosa relies crucially on iron for growth (Cox, 1986) [11].
Pyocyanin is a natural derivative of the nitrogen-containing heterocyclic compound called phenazine. It is a redox active secondary metabolite and soluble in chloroform. At the genetic level, 7 genes, namely phz C, D, E, F, G, M and S, were identified to be responsible for the biosynthesis of pyocyanin in P. aeruginosa. These loci were found in all Pseudomonas spp [12,13,14]. The phz M and phz S genes were found to be the main genes responsible for converting phenazine-1-carboxilic acid to pyocyanin. However, little information about the enzymes encoded by the phz M and phz S genes is known.
The antibacterial effect of pyocyanin was first reported in 1940, when its effect on the inhibition growth of Escherichia coli was tested, the pigment was named colicin back then [15]. The purified form of pyocyanin exhibited a concentration dependent bactericidal effect [16]. The mechanism behind the antibacterial effect of pyocyanin was investigated and it was concluded that pyocyanin prevents the cells from performing their active metabolic transport process by interacting with the cell membrane respiratory chain [17]. When E.coli was treated with pyocyanin, the oxygen supply to these cells was depleted, in addition to H2O2 production, and diversion of the electron flow, causing toxicity to E. coli cells [18]. Pyocyanin was also reported to have an anti-staphylococcal effect, for pyocyanin extracted from P. aeruginosa isolated from the sputum of Cystic fibrosis (CF) patients inhibited the growth of Staphylococcus aureus [19]. Pyocyanin also showed a bactericidal effect against Salmonella Paratyphi and E. coli [20]. It is now accepted that pyocyanin accounts for 90-95 % of the ability of P. aeruginosato inhibit bacterial growth. In fact, pyocyanin isolated from P. aeruginosa 4B strain, was shown to exhibit an antibacterial effect against food spoilage bacteria like Listeria monocytogenes and Bacillus cereus [21]. In addition, when pyocyanin from P. aeruginosaDSO-129 was produced in a low cost microbioreactor with high processing, it was shown to have an inhibitory effect against several microorganisms including S. aureus, Staphylococcus epidermidis, Bacillus subtilis, Micrococcus luteus and saccharomyces cerevisiae [22].
The inhibition of fungi like C. albicans by pyocyanin was also clinically proven in patients with lung infections, as infection by C. albicans recurred after pyocyanin suppression [23]. Furthermore, Aspergillus fumigatus and Candida albicans, isolated from the sputum of Cystic fibrosis patients, were also inhibited by pyocyanin isolated from P. aeruginosa [24].
This study aimed at determining whether pyocyanin had the ability to inhibit the growth and/or the formation of biofilms by different Lebanese clinical isolates.
Materials and Methods
Bacterial isolates
The bacterial isolates used in the study were clinical isolates courteously provided by the Clinical Microbiology Laboratory of the Lebanese American University Medical Center- Rizk Hospital (LAUMC- RH) and 4 isolates from a community health center referred to in the study as public isolates (PI). In total, the isolates tested were the following: 5 isolates of Escherichia coli, 5 isolates of Staphylococcus aureus, 4 isolates of Proteus mirabilis, 4 isolates of Klebsiella pneumoniae, 4 isolates of Enterobacter cloacae, 3 isolates of Citrobacter koseri,2 isolates of Staphylococcus saprophyticus, 2 isolates of Enterococcus fecalis, 2 isolates of Acine to bacter baumannii, and 2 isolates of Morganella morganii. The identity of the isolates was confirmed standard tests [25]. The identity of the Gram-negative bacilli was confirmed using the API 20E test strips (Biomerieux-France).
Aqueous extraction and purification of pyocyanin and testing for antibacterial effect
Aqueous extraction and purification of pyocyanin
pyocyanin was extracted using the method previously described by Wilson et al. (1987), [26] but as amended by Abou Raji El Feghali and Nawas (2018) [27]. Major amendments were related to media used to grow the organism, incubation and holding time and the addition of a step to the acid/base extraction, to optimize the yield of pyocyanin.
Antibacterial activity of pyocyanin
The antibacterial effect of the aqueous extracts was performed by using the well agar diffusion method using Mueller-Hinton agar (MHA) as recommended [28]. Using a sterile swab, the MHA plates were seeded with the organism taken from a saline tube containing the fresh test organism and adjusted to have a turbidity equivalent to that of a 0.5 McFarland standard. Using a cork borer, an 8.5 mm well was placed in the middle of the plate. The well was filled with increasing volumes of pyocyanin extract, from 200µl to 700µl [29]. The plates were then incubated at 35°C for 24 h, after which the of zones of inhibition of growth around the wells (whenever present) were measured using a caliper. The reported results were the averages of the diameters measured for each of the zones of inhibition of growth that surrounded the wells for each concentration of the tested extract for each test organism.
Methanol extraction and testing for anti-biofilm activity
Preparation of the methanol extract
In the extraction and purification procedure used in the study, [27] and in the final steps after the dissolution of pyocyanin crystals with chloroform, 0.05M HCL was added to the mixture and the red aqueous phase extracted and neutralized drop by drop by 0.05M NaOH until the blue color was recovered. Afterwards, methanol was added to the mixture making it 80% of the total volume.
Effect of the methanol extract on biofilm formation
Preparation of the bacterial isolates
From fresh agar plates, each of the test organisms was used to inoculate a 10 ml trypticase soy broth (TSB) tube with 1% glucose. The inoculated TSB tubes were left in the incubator at 37°C for 24 h, after which, the culture tubes were diluted 100 times with fresh media.
Effect of the extracts on biofilm formation
To test for the formation of biofilms by the isolates and possible inhibition of the process by the methanol extract of pyocyanin, a method very slightly modified from that used by Mathur et al. (2006) was used [30]. The methanol extract was added to specified test wells of the 96 well flat-bottom tissue culture plates and the plates were left to dry in the incubator under aseptic conditions. Upon drying, 200 µl of sterile TSB were added to all the wells of the plates with 10 µl of the diluted cultures (previous section) and incubated at 35°C for 24 h. The contents of the wells were then gently discarded by repeated soft tapping, after which the wells were washed with phosphate buffered saline (PBS, pH of 7.2) several times. Then, 0.2% sodium acetate was added to fix any biofilms that may have formed, and a 0.1% solution of crystal violet was finally added to stain the biofilms, when present. Excess stain was then removed with deionized water and the plates were left to dry. The optical densities were later determined by using a microplate auto-reader at 570 nm wavelength. To have a precise result, each of the test samples (and controls) was performed in 16 wells. The reported optical densities, in the study, were the averages of the 16 readings of each sample.
Results
The aqueous extract of pyocyanin showed clear antibacterial activity against a wide range of the bacteria tested. As is obvious from Table 1, of the Gram-positive isolates, all the 5 S.aureusisolates were inhibited at a volume of 200 µL of the extract, while the 2S. saprophyticus and 2E. fecalis isolates were also inhibited, however, one of each was inhibited by a volume of 200 µL, while the others by a volume of 300 µL. For all, however, the antibacterial effect was shown to increase with the increase of volume of the extract added. On the other hand, the results varied for the Gram-negative isolates, for whereas the growth of E. coli, C. koseri, and E. cloacae and A. baumannii isolates was clearly all inhibited either by a volume of 200 µL or a volume of 300 µl, (with a clear increase in antibacterial activity with increase in volume of pyocyanin tested), yet the growth of all the K. pneumonia, P. mirabilisand M. morganii isolates was not affected by the pyocyanin extract even with the maximal volumes of pyocyanin used in this study (Table 1).
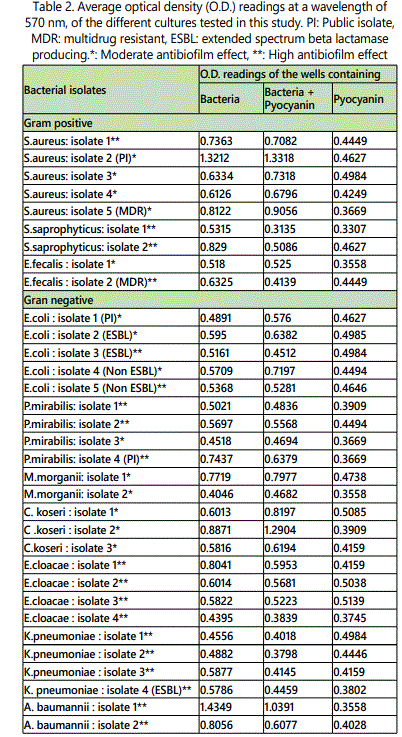
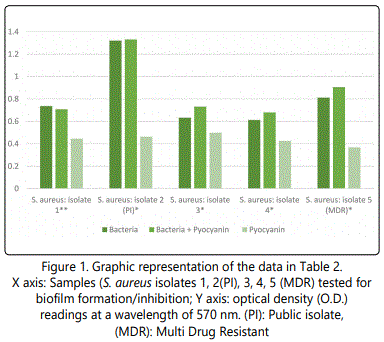
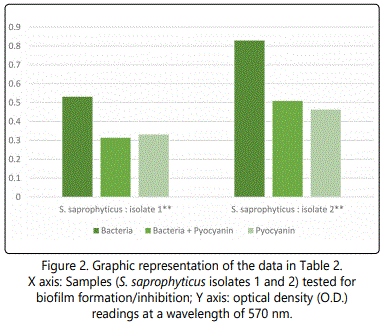
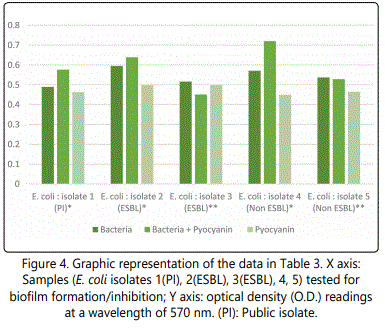
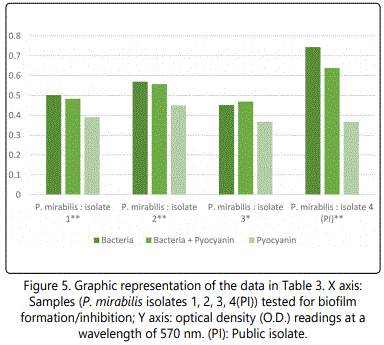
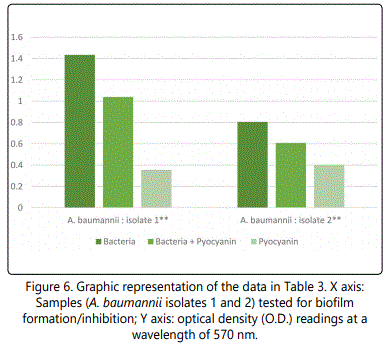
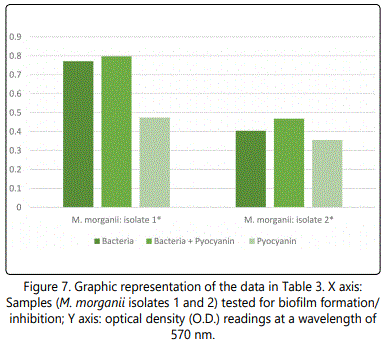
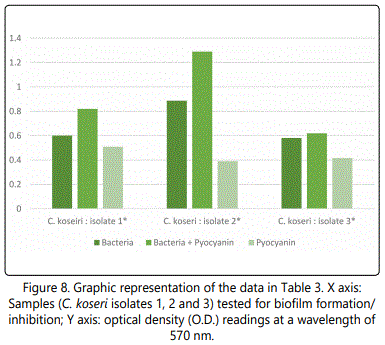
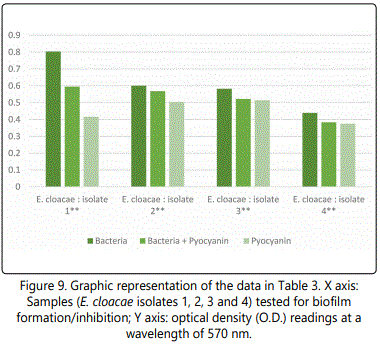
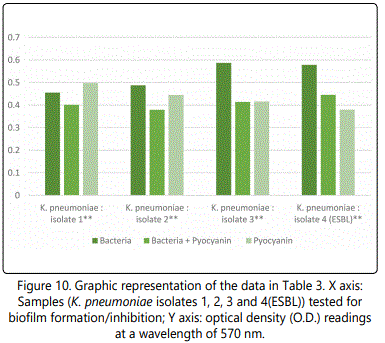
As to the ability of pyocyanin to inhibit the biofilm formation of the different isolates, Table 2 represents the optical densities read for each of the samples tested and their controls. The same results are also represented graphically for individual species in Figures 1 to 10. The results clearly indicated that all the clinical isolates were capable of forming biofilms on the bottom of the tissue culture plates. The power of the pyocyanin methanol extract to affect the ability of the isolates to form biofilms is also obvious. The pyocyanin methanol extract was highly effective in preventing the biofilm forming ability of all S. saprophyticus (Figure 2), A. baumannii (Figure 6), E. cloacae (Figure 9) and K. pneumoniae (Figure 10) isolates, 3 of the 4 P.mirabilisisolates (Figure 5), 2 of the 5E.coli isolates (Figure 4), 1 of the 2 E. fecalis isolates(Figure3) and 1 of the 5S. aureus isolates (Figure 1). All the other isolates in this study including the M.morganii and C.koseri isolates, however, showed a moderate antibiofilm activity (Figures 7&8).

Discussion
Previous reports noted the antibacterial and antifungal activity of pyocyanin [19,23,24,31,32]. The results of this study showed that the antibacterial effect of pyocyanin on the different Lebanese bacterial strains tested, was also evident. The Gram-positive organisms including S. aureus, S. saprophyticus and E. fecalis were all inhibited even using low concentrations of the aqueous pyocyanin (200 or 300 µL). A similar result was reported by Machan and his colleagues in 1991, where pyocyanin extracted from P. aeruginosa from cystic fibrosis (CF) patients exhibited anti-staphylococcal activity by inhibiting the growth of S. aureus. These results were obtained through the cross-streak test, well plate assay and growth of mixed culture [19].
Most of the Gram-negative bacteria tested in this study, namely E. coli, E. cloacae, C. koseri and A. baumannii, were also inhibited by low concentration of aqueous pyocyanin (200 or 300 µL). The antibacterial effect of pyocyanin on certain Gram negative bacteria was also previously noted and reported, and it was shown that pyocyanin had an antibacterial effect against E. coli and Acine to bacter isolates [31,32].
It was noted in this study, however, that pyocyanin, even at the higher volumes used did not in any way affect the growth of the K. pneumoniae, P. mirabilis or M. morganiiisolates tested (Table 1). Similar results were reported by previous studies. Sweedan in 2010, reported that K. pneumoniae and P. vulgaris were resistant to any antibacterial effect that pyocyanin may have, [31] while ElShouny and his colleagues (2011) also reported that K. pneumoniae was fully resistant to any antibacterial activity that pyocyanin may have demonstrated [32].
It is also worth noting from Table 1 that the antibacterial effect of pyocyanin, on the different isolates that were susceptible to it, was concentration dependent and increased with increasing concentrations of the pyocyanin added. The diameters of the zones of inhibition of growth increased with increasing concentration of pyocyanin. The effect of pyocyanin, as an antibacterial agent, on the Gram-positive bacteria was evidently much stronger than that on the Gram-negative bacteria as was clear from comparing the diameters of the zones of inhibition of growth of the two groups (Table 1).
The mechanism through which pyocyanin exhibits its antibacterial activity was previously studied and it turned out that pyocyanin interacted with the respiratory chain of the cell membrane preventing the susceptible bacteria from performing their regular metabolic processes [17]. Furthermore, pyocyanin was toxic to the E. coli cells by depleting the oxygen supply to the cell, producing H2O2 and diverting the regular flow of electrons [18].
After reviewing the literature, it is believed that this study is the first to demonstrate the ability of pyocyanin methanol extracts to inhibit the biofilm formatting ability of several clinically important bacterial strains (Table 2 and Figures 1-10). This finding is extremely important as one of the most challenging problems in antibacterial therapy remains the difficulty in targeting bacteria that are, enclosed within formed biofilms [33].
Conclusion
This study revealed that pyocyanin produced by P. aeruginosa has a powerful inhibitory effect on the bacterial growth and/or biofilm forming ability of the numerous clinically significant isolates tested. Such results could be supported by future studies on the rate, frequency and type of other infections in the presence of P. aeruginosa, like in the case of cystic fibrosis (CF) patients. Pyocyanin may turn out to be a valuable new addition to the currently existing medications as an antibacterial drug or a chemical used in the prevention of bacterial biofilm formation. Further studies are also needed to assess the safety of using pyocyanin on hosts and their microbiota.
References