Research Article
Human, Urban and Environmental-Induced Alterations in Mangroves Pattern along Arabian Gulf Coast, Eastern Province, KSA
1Department of Biology, Faculty of Science, Fayoum University, Egypt
2Department of Biology, Faculty of Science, University of Dammam, KSA
3Department of Biology, Faculty of Science, Damietta University, Egypt
*Corresponding author: Sameh A Amin, Department of Biology, Faculty of Science, University of Dammam, Dammam, Saudi Arabia, E-mail: saismail@uod.edu.sa
Received: October 24, 2018 Accepted: November 17, 2018 Published: November 26, 2018
Citation: Amin SA, Fouad MS, Zyada MA. Human, Urban and Environmental-Induced Alterations in Mangroves Pattern along Arabian Gulf Coast, Eastern Province, KSA. Madridge J Case Rep Stud. 2018; 2(2): 94-100. doi: 10.18689/mjcrs-1000124
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Significant changes have occurred at Arabian Gulf Coast of Saudi Arabia over the last three decades. The area of mangrove was reduced by about 55%. Thus the economic, social, and environmental value of mangroves must be assessed over short- to long-term scales and employ these assessments for the awareness rising at local communities. This study provides a preliminary assessment of the risks on mangrove vegetation; it will provide database to mitigate the tremendous pressures due to coastal development and urban activities. The effects of human development on the mangrove plant cover in Eastern Region of KSA were recognised, during 2013 to 2016. The mean variations of physio-chemical characteristics in water and sediment were recorded. With regard to water analyses including; nitrogen, phosphorus, TSS, TDS, BOD, and turbidity were evaluated. On the other hand, for sediment: TDS, nitrogen, phosphorus, sulphate and total organic carbon were assessed. Moreover, the growth parameters: plant height, and size index, of Avicennia marina were recorded and estimated. The results revels that the mangrove communities subjected to severe degradation and depletion. It concluded that human impact and urban developments have exerted drastic effects on the coastal ecosystems and its environments.
Keywords: Mangrove; Avicennia marina; Size Index; Sediment; Urban Development; Human Impacts.
Introduction
Mangroves alleviate devastating effects of erosion, storm surges and flooding of coastlines [1]. Moreover, the importance of coastal intertidal mangrove habitats comprises their contributions in increasing the sedimentation rate [2], acting as a physical and biogeochemical barrier for contaminants attacking coastal estuaries and other water bodies [3,4]. Mangroves also helping in the accumulation and partitioning of trace elements in the rhizosphere [1]. Considered as woody plants mangroves inhabit intertidal zones with high salinity [5] and can tolerate a wide range of salinities under natural conditions [6]. Also mangroves have wide ecological amplitude ranging from tropical to temperate regions across all continents and dominate large extents of shorelines, estuaries and islands in tropical and subtropical regions worldwide forming biologically important and productive transitional coastal ecosystems [7-9]. As mangroves are considered as assemblages of trees and shrubs, they sustain both ecological and economical services in ecosystems [10], and playing an important role in both biogeochemical cycles and economic activities [11]. About 80 species of true mangrove trees/shrubs are recognised, from which around 50-60 species make a significant contribution to the structure of mangrove forests. Species diversity is much higher in the Southeast Asian Region, where approximately two-thirds of all species are found, while approximately 15 species exist in Africa and 10 species in the Americas [12]. It is known that more than 90 % of the world's mangroves are located in developing countries [13]. The trees of several genera are economically valuable for timber or fuel wood, especially Rhizophora species. Since, more than one-third of the global human population lives along coastal areas, their long-term sustainability depends on the coastal ecosystems [14]. However, human activities and interventions within and near these mangrove areas have led to their degradation and the resources there in [7,12]. Large areas of mangrove have been converted into fishponds, salt ponds, agriculture and coastal projects [15]. Over the globe, due to disturbance of species distribution, mangroves have been disappearing at an alarming rate worldwide by an annual rate of 1-2% [16]. The loss of mangrove forests has increased from regions of highly anticipated rise in global temperature [17]. Thus, mangroves may vanish if the destruction of their ecosystems continues repeatedly [13].
Mangrove plant covers are found along some coastlines of the arid Arabian Peninsula [18]. They are present in the form of fragmented stands in many tidal areas on the Red Sea and the Arabian Gulf coast, south of latitude 26°C north. They consist mainly of Avicennia marina trees. On the coast of the Red Sea, Avicennia marina is accompanied by a few of Rhizophora mucronata, however it is very rare in Saudi Arabian Gulf [11]. Mangrove ecosystems are limited along Arabian Gulf and they are confined to Dammam area (Taraut Bay), with well-developed communities consisting of Avicennia marina. The inter-tidal mangrove environment of Eastern Region of Arabian Coast is very important as it supports the local fishing activities, nursery grounds for many fish, crustaceans and shellfish species, as well as being central for ecotourism activities. Also due to the consistently diminishing erosion resistance, the extent of mangrove plant cover along the Saudi Arabian Gulf has been considerably decreased. Moreover, in the Arabian Gulf area mangrove ecosystems have been principally affected by the large oil spills from the Gulf War [19]. It is also threatened by the expansion of human settlements, the boom in commercial aquaculture, the impact of tidal waves and storm surges. Noticeably, in Taraut Bay it has exhibited drastic reduction due to major stressors including landfilling, dredging, coastal development, solid and liquid waste disposal [20].
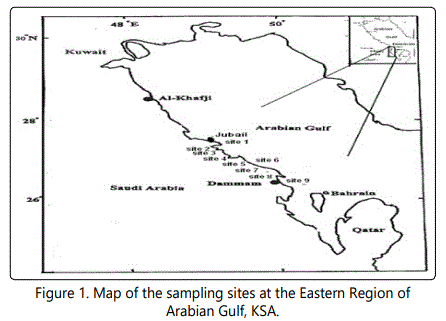
It is not too late to renew the loss in productivity of the mangrove areas, and their plant cover can be rehabilitated and maintained [21]. The present study conducted to frame the human impact assessing the physico-chemical parameters on mangrove vegetation in Dammam area (Figure 1), especially at Taraut Island. In addition, the current work is an attempt to demonstrate threats and difficulties that are facing mangroves in their natural habitats. Also, this work outlines the severity of ecological implications that disturb the coastal ecotone where mangrove thrives.
Materials and Methods
Study site
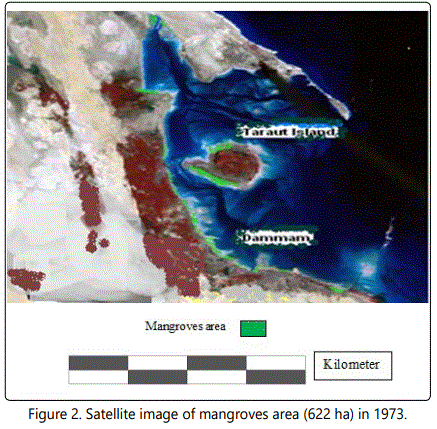
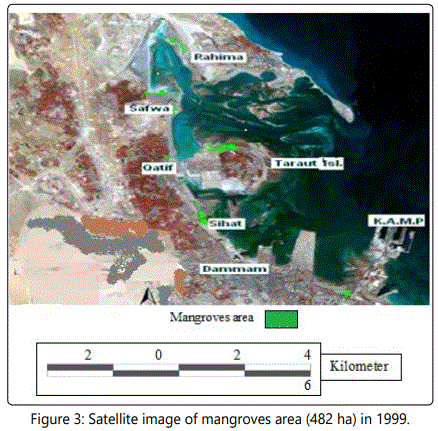
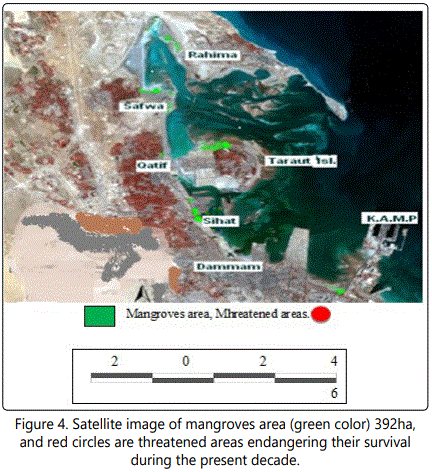
Satellite, Google Earth, pictures, images and geographic information systems provide collectively useful tools to detect and map the temporal variation in mangroves coverage [22, 23]. For this purpose the temporal changes in the geographic distribution of mangroves along the Arabian Gulf Coast at Dammam Region-El QatifCity, Taraut Island-(Figure 1) have been studied by conducting the historical Landsat Multispectral Scanner (MSS) and Landsat Enhanced Thematic Mapper (ETM) adopted by Riaza A et al. [22]. New changes in these maps and images were made in the present work through field studies (Figures 2-4).
Field measurements
a) Vegetation: Nine locations were selected across mangrove forest (Figure 1). Three transects were outlined and laid out in such a way to represent the variations of mangrove trees at each site. The following criteria were estimated namely: abundance and growth parameters (the plant height, size index, leaf area, number of main and lateral branches, number and height of aerial roots and number of seedlings/m2 ) of mangrove were measured at each site to evaluate the growth rate.
b) Water and sediment sampling and analysis: Across a distance of about 8 to 10 km along the Arabia Gulf Coast of Eastern Region, KSA (Figure 1), monthly visits were arranged to the studied area throughout the period from January 2013 to January 2016 collecting water and sediment samples from the same experimental locations. Surface water and sediment were gathered randomly from a depth of 0-15 cm. Surface water and sediments were analyzed to determine both physical and chemical characteristics. The concentration in water and sediment are expressed in mg/Land in mg/kg dry weight respectively.
The experimental results were extended to measure pH and turbidity of the water in-situ (in duplicate); using the electrometric method for pH and the Nephelometric method for turbidity as per standard methods [24]. The biological oxygen demand (BOD) was determined following the method described in a study by American Public Health Association [25], while TDS is measured according to standard method of American Public Health Association [24].
Consequently, the collected sediment samples were air dried and then crushed for further analysis. A 5 g of fieldmoist soil was thoroughly mixed with 25 ml distilled water in polyethylene centrifuge tubes and placed on a spinning wheel for 2 h on termination of the shaking the soil-water slurry was left to settle for 10 min and its pH was measured using an Orion 290A pH meter [26]. Also, the sediment organic matter (TOC) was determined according to American Public Health Association [24].
Phosphorus concentration was estimated using a colorimetric assay as described by Reef R et al. [27]. Also the method described by Marinho CC et al. [28] was used to quantify the nitrogen content in the soil samples. It has to be noted that this method is a modification of the standard Kjeldhal-N method. Furthermore, soil samples were analyzed for Sulphates estimation according to the method adopted by Jackson ML [29] by titration against BaCl2 in presence of tetrahydroxy-quinone as indicator.
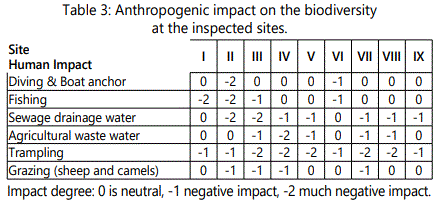
c) Human impact assessment: As Tarout Island is predominantly considered as one of the most ancient sites that were inhabited by humans, moreover, the Island had a significant role in trading purposes in the entire Arabian-Gulf region (https://en.wikipedia.org/wiki/Tarout_Island). Since the island has a legacy of severe human-induced environmental degradation over its existence, therefore the effect of landfilling, dredging, coastal development and solid waste disposal are presently studied regarding mangrove ecosystem. Therefore, it is mandatory in this present work to quantify anthropogenic disturbance and fouling in terms of degrees (0,-1,-2) on the biodiversity of the sites under investigation.
Statistical analysis
Analyses of variance one way ANOVA for the water, sediment and mangrove abundance (one-way and two-ways) were carried out. The same analyses were done for mangroves biodiversity. This analysis showed a strong significant difference for both one-way (sites) and two-ways (sites and parameters), P<0.05: 0.01.
Results
The recorded data in tables 1 and 2, clarifies the values of physico-chemical characteristics of water and soil at the investigated sites of Arabian Gulf coast in Saudi Arabia, respectively. The average measured values of pH are within the alkaline range. However, total dissolved solids (TDS) exhibits normal concentrations with relatively steady rates in most samples, site I exhibits the maximum TDS in water (44500 ± 4000 mg/ L). Meanwhile, site V showed the highest total suspended solids (TSS) magnitude (5.4 mg/L) followed by site VI. The rest of specimens showed a descending trend.
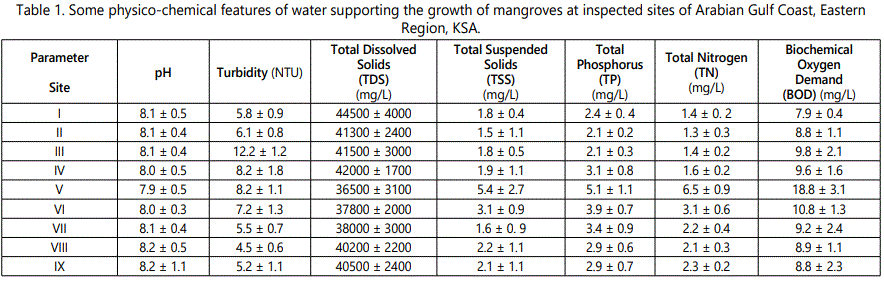
The turbidity measurement was found to have the highest values in site III (12.2 NTU) followed by sites IV and V when compared to other locations. Since Phytoplankton sediments from erosion and re-suspended sediments arising from agitation of the bottom sediments, comprises some detritus particles which are light, remain suspended in water. Table 3 exhibits a list of anthropogenic actions including waste discharge, trampling is the more effective activity and has much negative impact followed by sewage water drainage. Site II is the most affected locality, wherever it affected negatively by all investigated human activities (Table 3).
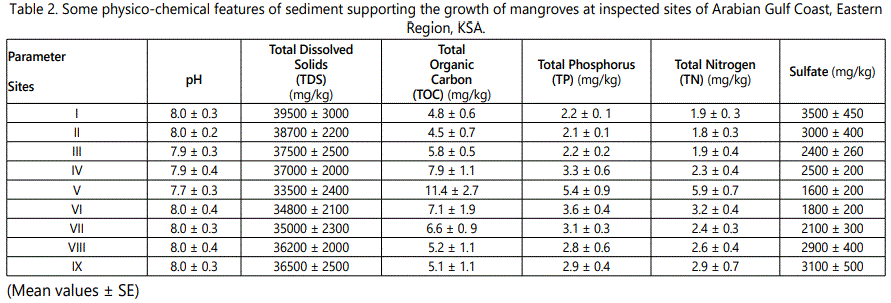
Regarding the total phosphorus and nitrogen, biochemical oxygen demand BOD and total organic carbon TOC, site V followed by site IV recorded the maximum values when compared to other sites (Table 3). Site I showed the lowest concentrations. For all the investigated sites, the amount of sulfates (SO42-) exceeds 1600 mg/kg, a result that is emphasized by Reddy and D'Angelo [30] stating that the minimum value of the sulfates in the coastal area must not be less than 2 ppm. On the other hand, the acceptable range of nitrogen in the soil is between 10 ppm to 60 ppm. Hence, nitrogen is lower than the normal range over all sites. The distribution of P and N2 are associated with organic matter. Apart from site V, variations recorded for the sites are relatively small, and may be associated with contrasting sedimentation patterns, which is in accordance with [28].
Table 3 and figure 5 particularly at site V could be referred to the increased levels of organic carbon. TOC levels imply that the sediments inside Mangrove forest could retain organic matter.
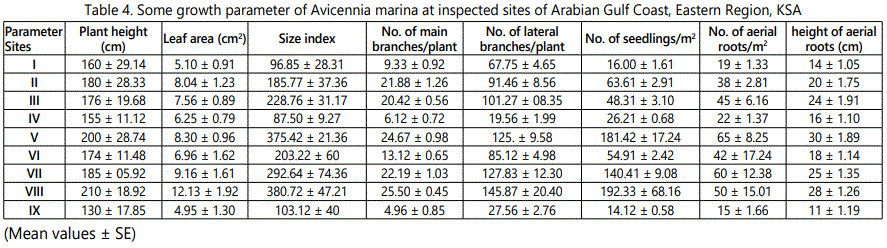
Investigation of table 4 showing that growth parameters of Avicennia marina growing in studied areas affected by the physico-chemical features of both water and sediments, and by anthropogenic impacts of human activities.
Discussion
According the high primary productivity of mangroves, it turnover rates of organic matter and the permanent exchange with the terrestrial and marine ecosystems [31]. Mangroves enable and supports biological life in agreement with previous studies [32,33]. Mangroves are well known for their halophytic characteristics [34], which allow them to survive in high salinity through certain mechanisms of salt tolerance [35]. Several studies have reported that Avicennia marina is largely predominant in high saline environments, where salinity is shown to be ≥ 25 ppt during most of the months of the year [36, 37]. Moreover, Avicennia marina is a facultative halophyte having various adaptations for hyper saline environments [38,39], urban runoff and fishing yield to an aggravated problem. Guhathakurta and Kaviraj [40] demonstrated similar deductions. The largest values of total phosphorus and nitrogen, biochemical oxygen demand BOD and total organic carbon TOC in sediment could be referred to the increased concentration in water [41]. Sulfur is an essential chemical component which is used as sulfate (SO42-) for the structure of the plants and different biological processes [42]. Turbidity could be employed to provide an estimation of total dissolved solids.
Additionally, the decayed litter released nutrients into sediments, which would contribute to the increase of organic matter in the sediments. Consequently, Mangrove plants improve the sediment fertility and promote the physicochemical properties of the intertidal habitat, which would influence the biogeochemical processes of other elements in the sediment [43]. During Mangrove succession, the diversity and biomass of the plant community increase; therefore, the matured Mangrove community provides an abundance of sediment biogenic matter, such as SOM, TC, TN, TP and TK [44].
Plasticity of mangrove seedlings in environmental conditions is common but has mainly been related to other a biotic factors such as light or water availability [45]. Owing to the richness of some investigated locations with nutrients, hence the high abundance and growth parameters “plant height, size, number of main and lateral branches” of Avicinia marina at sites V, VII and VIII were noticed as compared to the other sites, while the lowest density was obtained at sites I, IV and IX. Human impact was recorded at all sites, where urban development was clear in sites IV, V, VI and VIII [46].
Table 3 and Figure 5 dealing with the human impact on the mangroves in the studied sites through illustrating the quantity of waste water drained in the sites under consideration. Thus, the elevated amount of nutrients in water and sediment may be attributed to the increase of sewage and agricultural drainage water at these sites, wastes from ships and boats and in addition to the decomposition of dead plants, particularly at site V that could be referred to the increased levels of organic carbon. TOC levels imply that the sediments inside Mangrove forest could retain organic matter.
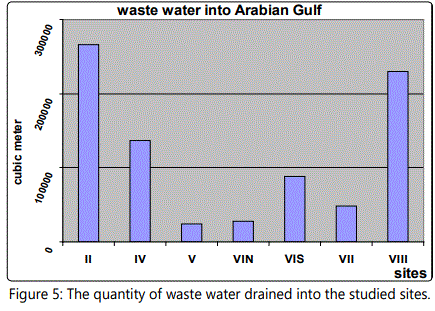
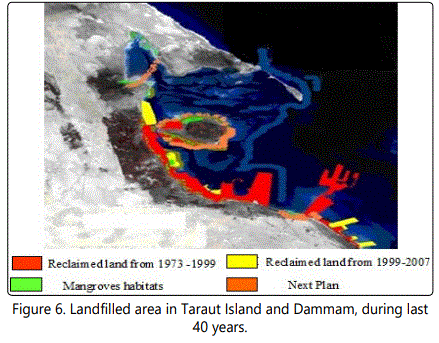
Significant changes have occurred in Saudi Arabia Gulf coast over the last three decades. Figures 2 and 3 showed the Satellite image of mangroves area (622 ha) in 1973 which is reduced to an area of 482 ha in 1999 [46]. Many aspects of development were recorded in Dammam and Qatif regions that negatively affect the mangrove plant cover. Figure 4 shows the last urban disturbance on mangrove which results in plant cover reduction from 482 ha to 390 ha during 1999 to 2009. In a close observation, figure 6 shows the landfilling in the studied area during the last four decades. There is a negative impact on the mangrove occurrence as a result of the coastal development that include dredging, filling, and other activities like oil pollution and drainage water (Figure 5).
Conclusion
The present work emphasizes the ability of Avecinnea marina to overcome contamination and human disturbances in the ambient environment. The economic, social, and environmental value of mangrove must be assessed for awareness rising of local communities. However, greater losses in fringe mangroves and an increased area of basin mangroves can be related to sea-level rise and increased mean air temperatures that in turn related to current climate change [47]. Climate change is a futuristic problem creating stress to the entire biosphere. The overexploitations of mangrove forest and oil pollution are considered the main destruction factors in the Arabian Gulf [49]. Wide distribution of mangrove is ascribed to its adaptation resilience. A conducted trial In Saudi Arabia, remote sensing data was used to locate suitable sites for mangrove plantation along the Red Sea Coast [49].
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References