Research Article
Assessment of the Topical and Systemic Effects of Omega-3 on Oral Mucosal Wound Healing in Albino Rats: A Histopathological and Biochemical Study
1Lecturer of Oral Medicine and Radiology, Faculty of Dentistry, Pharos University, Alexandria, Egypt
2Lecturer of Oral Pathology, Faculty of Dentistry, Fayoum University, Cairo, Egypt
3Professor of Oral Pathology, Faculty of Oral and Dental Medicine, Cairo University, Cairo, Egypt
*Corresponding author: Mervet Moussa, Professor, Departments of Oral Pathology, Cairo University, Egypt, E-mail: drmervet@yahoo.com
Received: September 4, 2017 Accepted: April 2, 2018 Published: April 9, 2018
Citation: Basha S, ElRefai SM, Moussa MM. Assessment of the Topical and Systemic Effects of Omega-3 on Oral Mucosal Wound Healing in Albino Rats: A Histopathological and Biochemical Study. Madridge J Case Rep Stud. 2018; 2(1): 26-31. doi: 10.18689/mjcrs-1000107
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The use of herbal medicine is increasingly becoming more accepted in treatment of many inflammatory conditions. Omega-3 is recently introduced as an effective therapeutic agent and a powerful anti-inflammatory agent, which promotes whole-body wound healing including ulcers as it has an important role in modulation of proinflammatory cytokine production. This study was conductedto evaluate and compare the effect of topicalapplication and systemic administration of omega-3 on the healing of oral mucosal wounds which wereexperimentally induced in albino rats. The experiment comprised 45 albino rats which were divided into 3 groups (n= 15 per group); control group not receiving any treatment(C) group (L) receiving topical application of omega3, and group(S) receiving systemic omega3; 5 rats from each group were sacrificed at day 2, 4 and 8 respectively. Tissue samples were obtained and prepared for histological and biochemical evaluations. The data obtained were analyzed using one way ANOVA and Post Hoc Tukey statistical analysis with the previously performed tests of normality and homogeneity. The biochemical results showed significant improvement regarding TNF- α and IL8-levels after 4 and 8 days in both groups compared to the control. The topical omega3 group showed better results than the systemic with significant differences at 4& 8 days inTNF- α while for IL8 the difference was only significantafter 8 days. The histological results revealed severe epithelial degeneration and inflammation in the lamina propria after 2 days whether using local or systemic treatment however increase reepithelialization gradually increased at 4 days and an even thickness of the epitheliumwas noted by the end of day 8.
Conclusion: Omega 3 greatly enhanced ulcer healing and showed superior effects using topical application.
Keywords: Oral ulcers; Omega 3; Interleukin 8; TNF.
Introduction
Ulcers are among the most frequent lesions encountered in the oral cavity, they may be manifested as either solitary or multiple lesions affecting skin and/or oral mucous membrane [1].
An ulcer is generally defined as a break in the skin with loss or discontinuity of surface epitheliumand can also be defined as a local defect due to disruption in mucosal integrity. The exact cause of ulcersremains unclear, nevertheless many factors have always been blamed including primarily trauma, moreover chemicals, infections, cancers, radiation and certain therapeutic drugs [2].
Ulcers can be classified into either primary lesions, with early manifestation such as erosion, or secondary if they result from subsequent rupture of vesicles and bullae. The exact pathogenesis of ulcers differs vastly according to their etiology [3,4].
Ulcers are painful sores that cause discomfort, the goal of its treatment is to relieve symptoms; therefore, finding suitable drugs with fewer side effects is the concern of many researchers. Steroids (topical and oral) and mouthwashes are usual treatments for oral ulcers; however, systemic absorption of local steroids can have undesirable influences on the immune system and may lead to secondary infections. More complex processes for such lesions are seen in some autoimmune diseases, where a number of different genetic mutations take place [5,6].
In recent years, Omega 3 has received attention being rediscovered as an effective therapeutic agent and a powerful anti-inflammatory, which promotes whole-body wound healing including ulcers [5,7]. It is naturally found in common diet as in cod liver oil, fish oil, marine animals with a high amount of fat, such as tuna, and plant oils from walnuts. Omega 3 fatty acids are bioactive lipids making an important part of cell membranes, thus they modulate membrane protein function, cellular signaling, and gene expression. Considerable interest has been focused on the potential health benefits of omega-3 fatty acids on many diseases [8,9].
It was also recently discovered that (omega-3 fatty acids) helped defend against ulcers on multiple fronts by augmenting both defensive and offensive factors thus enhancing clearance of inflammation within the lesion promoting tissue regeneration [8,10].
Tumor necrosis factor α (TNF-α) is one of a group of proinflammatory cytokines that appears rapidly after infection and injury. It has a pivotal role in withstanding pathogenic invasion [11]. Similarly IL-8 referred to as neutrophil chemotactic factor (NCF) and neutrophil activating factor (NAF) is an important mediator of host response to injury and inflammation. It acts as chemo-attractant for T cells and are significantly increased in patients with oral aphthousulcers [12].
Material and Methods
Animals: 45 adult male Albino rats weighting (200-250 g) obtained from the animal house of Medical Research Institute, Alexandria University.were used in the study. Throughout the study the animals were kept at the animal house of faculty of Dentistry, Pharos University in polypropylene cages, 10 rats each fed normal diet, and water add libitum.The room temperature was about 22-24°C and the animals were exposed to 12:12 hours light dark cycles. The experimental research protocol was approved by the Ethics Review Board of Faculty of Dentistry, Pharos University Number: 110 /2017
Ulcer induction: All experimental rats were then subjected to an induced ulcer on their palatal oral mucosaeas follows:
The rats were fixed on their backs and anaesthetized with intravenous pentobarbital injection 50 mg/kg). In order to create an ulcer, a round 5.5 mm in diameter filter paper was soaked in 15 ml of 50% acetic acid and pressed onto the palatal mucosa for 60 seconds,
Animals were then divided into 3 groups (15 rats in each group).
Control group(C): rats with induced ulcers not receiving treatment.
Local application omega3 group (L): a swab was soaked in omega-3 soft gelatin capsules 1000mg (SEDICO-EGYPT) was packedon the ulcer throughout the study period [7].
Systemically administered omega3 group(S): omega-3 with a concentration of600mg/kg body weight was guided into the stomach by gastric gavage twice daily throughout the study period [7].
Biochemical Evaluation
Excised palatal mucosal tissues obtained from the three experimental groups of rats were directly dissected and homogenized in appropriate buffer and then centrifuged, according to the instructions of the biochemical assay. The levels of TNF-α and IL-8 were measured using an enzyme-linked immunosorbent assay ELISA commercial kit (R&D Systems, EUA). Statistical significance of differences between the experimental groups was evaluated by one-way ANOVA. When multiple comparisons were performed the significance of differences was evaluated by the Post Hoc Test (LSD) P< 0.05 was accepted as significant.
Histological evaluation
Histological samples were collected on post-injury days 2, 4, 8 and each wound was excised maintaining approximately 3mm of normal palatal mucosa around the incision, specimens were then processed for paraffin embedding and longitudinal serial sections were cut to a thickness of 6 um. The sections were mounted on glass slides then stained with hematoxylin and eosin. Two independent examiners evaluated the specimens in a blind fashion in order to estimate the tissue response in the areas adjacent to the created ulcer and compared it with the normal tissues
Statistical Results
On the basis of ANOVA one way analysis test the level of TNF-α, after 2 days showed no significant difference between the three studied groups(. p1: p) value while after 4 and 8 days significant difference existed with ANOVA test (p2: p) value when comparing between the three studied periods (Table 1).
Concerning IL-8, significant difference existed between control and systemic groups and between control and topical groups after 2 and 4 days, but no significant difference existed between systemic group and topical group. However after 8 days significant difference existed between the three studied groups. p1: p value for ANOVA test comparing between the groups, p2: p value.
Post Hoc Test (LSD) applied with repeated measures for comparing between Control and Systemic, p3: p valueand also for comparing between Control and Topical, p4: p value and again between Systemic and Topical (Table 2).
Regarding the percentage of change of TNF-α levelwithin each group, significant decrease occurred from 2 to 4 days and from 4 to 8 days, which amounted to as high as 70% in the topical group (L) from 2 to 4 days while in the systemic group(S) it decreased only by 58% and the least amount of decrease was seen in the control group© and was equal to 38%.
Concerning to the percentage of changes in IL-8level within each group, significant decrease occurred from 2 to 4 days and from 4 to 8 days with the greatest decrease in the topical group (L) from 2 to 4 days by 60% while in the systemic group(S) it decreased by 50% and the least amount of decrease was seen in the control group(C) and was equal to 28%. These differences in IL-8 were statistically analyzed using Post Hoc (LSD) with repeated measures for comparing between 2 days and 4 days (Table 2), between 2 days and 8 days comparing between 4 days and 8 days (Table 3) respectively.
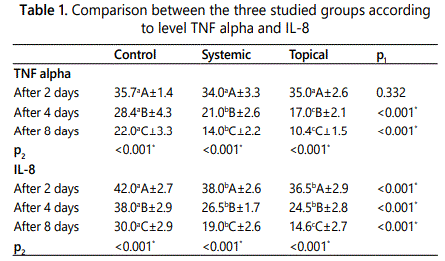
Means in the same raw followed by the same small letter were not significantly different at p<0.05.
Means in the same column followed by the same capital letter were not significantly different at p<0.05.
*: Statistically significant at p ≤ 0.05
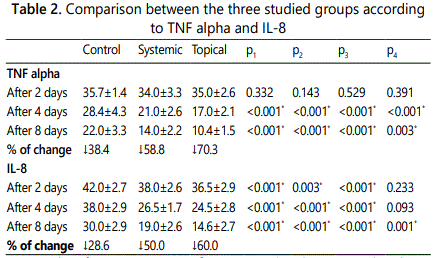
p1: p value for ANOVA test for comparing between the three studied groups
p2: p value for Post Hoc Test (LSD) for ANOVA with repeated measures for comparing between Control and Systemic
p3: p value for Post Hoc Test (LSD) for ANOVA with repeated measures for comparing between Control and Topical
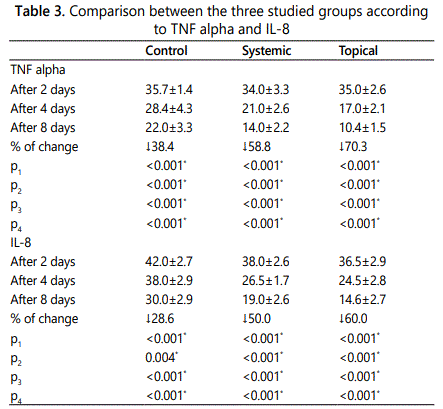
p4: p value for Post Hoc Test (LSD) for ANOVA with repeated measures for comparing between Systemic and Topical
*: Statistically significant at p ≤ 0.
p1: p value for ANOVA test for comparing between the three studied periods
p2: p value for Post Hoc Test (LSD) for ANOVA with repeated measures for comparing between 2 days and 4 days
p3: p value for Post Hoc Test (LSD) for ANOVA with repeated measures for comparing between 2 days and 8 days
p4: p value for Post Hoc Test (LSD) for ANOVA with repeated measures for comparing between 4 days and 8 days
*: Statistically significant at p ≤ 0.05
Histological Results
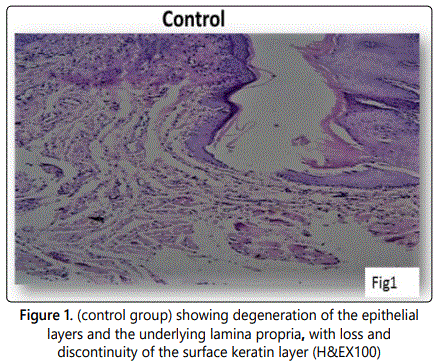
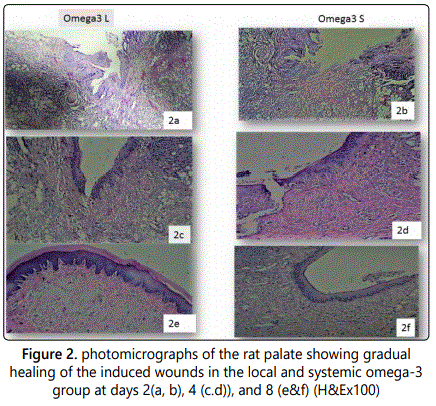
The experimentally produced ulcer of the control group showed a wound of considerable size with degenerated surface epithelial cells and remnants of degenerated surface keratin layer. The lamina propria and submucous layers were heavily infiltrated with inflammatory cells. (Figure 1a), (wound of the control group was evaluated only once and was not followed over the entire course of healing). The omega 3 treated groups both local(L) and systemic(S) showed epithelial degeneration with a deep ulcer formation and complete absence of the covering keratin layer (Figures 1b & c). After 4 days, the locally treated group(L) showed a remarkable reduction in the wound size with an intact epithelial layer lining most of its depth while the systemic treated group(S) revealed only slight degree of regeneration of the keratinized stratified squamous epithelium of the hard palate (Figures 1d & e) In both groups, moderate numbers of inflammatory cells were also noted. After 8 days both the locally treated group (L) and systemic group(S) showed an even thickness of the ulcer region and complete regeneration however the locally treated (L) groups presented full reepiselialization of epithelial rete ridges covered by a well keratinized surface and connective tissue papillae of normal thickness (Figures 1f & j) inflammatory cells were also noted however minimally infiltrated the sub epithelial layers of the locally treated group. Compared to those of the systemic (S) group.
Discussion
Oral ulcers are among the most common manifestations of oral diseases. Being painfuland annoying, treatment and management of oral ulcers is of utmost importance. Application of new treatments that are more patient compliant, less expensive, easy to use, readily available and with minimal side effects would be helpful [7].
Omega-3 polyunsaturated fatty acids are one of the best prescribed therapies for such conditions, owing to their clear role in early wound epithelialization, and inhibition of many inflammatory responses reported by various studies [13].
Experimental studies have found gastrointestinal benefits of omega 3 and has been shown to decrease the offensive acid-secretion, significantly increasing the activity of antioxidant enzymes and lowering lipid peroxidation in the rat gastric mucosa [14].
Its anti-inflammatory properties arise from processes such as the partial replacement of arachidonic acid in cell membranes. There is also a reduction in the chemotaxis of monocytes and neutrophils, modulating the inflammatory response in its earliest stages [15].
Tumor necrosis factor-alpha (TNF-alpha) also known as cachectin is an inflammatory cytokine and apoptotic molecule that is recognized as a crucial contributing factorin inflammation and fibrosis. While beneficial in host defense to pathogens, in excess, TNFα is associated with host damaging effects. Impaired healing in animal models and age-related delayed healing of acute human wounds exhibit raised local and systemic levels of TNFα [16,17,18].
Omega-3 has a protective action against the damaging effects of TNF-α, Khosroshahi et al reported in their study onhemodialysis patients a significant reduction of TNF-α level after two months of omega-3, they concluded that omega-3 fatty acids decrease the production of classic inflammatory cytokines as well as the expression of adhesion molecules involved in inflammatory interactions between leukocytes and endothelial cells [19].
IL-8 is a cytokine that plays an important role in inflammation and wound healing. Basit et al [20] reported that in many cell types, IL-8 has a capacity to recruit T cells as well as nonspecific inflammatory cells into sites of inflammation by activating neutrophils. Furthermore, IL-8 stimulates deposition of collagen I and other proteins during wound healing in vivo as confirmed by Matsuyama et al [21] who reported in their study on fiftychronic obstructive pulmonary disease (COPD) patients that omega 3 had a significant anti-inflammatory role in decreasing IL8 and TNFα levels [20,21].
Despite this weight of evidence and the considerable current use, there is still a need for further researches, therefore this study was conducted to assess the topical and systemic effects of omega-3 on mucosal wound healing regarding oral ulcers. The results of the present study regarding the biochemical analysis showed that there was a significant decrease in inflammation represented by the levels of IL8 and TNF either with the local or systemic omega 3 supplementation after days 2, 4, and 8, with a gradual decrease from one period to the other, and this was in agreement with Maryam elsadat et al 7 who reported in their study on rats (palatal ulcers), high scores of inflammation on day 2 and 4 in control group in comparison with the consecutive days while lowest inflammatory scores on days 6 and 8. These findings were also confirmed by This et al 23 and Kang et al 24 who showed decrease in TNFα in their reports.
The fact that omega-3 fatty acids play an important role in the regulation of inflammation can be explained through multiple pathways including, induction of several potent antiinflammatory lipid mediators, suppression of nuclear transcription factors activity, and reduction of the pro-inflammatory enzymes and cytokines [7,23,24].
The histological results of the present study, showed severe signs of epithelial atrophy with absence of keratin layer and inflamed underlying connective tissue. With local and systemic omega 3 application, there was a gradual reduction in inflammation, increase in epithelial and connective tissue regeneration, appearance of keratin layer, and finally high vasculature, all occurring gradually throughout the period of 2,4, and 8 days, giving the best repair effect at the end of the treatment period.
These results were in accordance with Maryam elsadat et al 7, where they showed that by using omega 3, the greatest reepithelialization being observed on days 6 and 8 of their study, and that the fibroblasts significantly increased throughout the whole period and promoted tissue regeneration, advocating that these fibroblasts produce PGE2, IL-6, and TNF-a, which have a role in collagen synthesis and in turn wound closure. Similar results were reported by Hankenson et al. 25 [7,25].
On the other hand ArzuGercek et al. 26, explained the reepithelialization through monitoring TGF-β levels, this growth factor inhibitor, which plays an important role in the healing process together with platelet derived growth factor (PDGF), they stimulate fibroblast chemotaxis, collagen production, and inhibit collagen degradation, thus, promoting fibrogenesis. This was confirmed by other studies that showed that the level of TGF increases gradually, being highest on day 6 of their study period, making tissue regeneration and healing more pronounced as time passes [7, 26].
Conflicting to these results, Cardoso et al [27], reported in their research using omega 3, 6, and 9, that omega 3 treated group showed tendency to a delay in wound closure in the first 10 days, which disagrees not only with our results but also with other authors who observed faster wound repair after administration of codfish oil. This difference could be explained by the fact that codfish oil has several components or concentrations that may influence healing, while we used purified preparations [27].
In consistence with Maryam elsadatet al 7, the results of the present study showed that there was a significant difference between the local and systemic supplements which was in favor of the local form regarding the decrease in inflammation and the better and faster wound healing.
These results were also supported by Terkelesn et al 28 who reported that omega 3 topical application significantly accelerated both the epithelial and vascular component of healing compared to the systemic supplement being clarified throughthe fact that systemic supplements need more time to heal the wound since they reach through blood unlike the direct effect that the local forms offer [7,28].
Conclusion
The findings of this study indicate that omega-3 fatty acids (L and S) can promote healing of oral ulcers in animal models where the topical application being more effective than systemic administration. The results showed wound size reduction, increase in fibroblastic activity, reepithelialization, and granulation tissue formation. To sum up, the use of omega-3 fatty acids is safe, practical, and could be an alternative treatment for oral ulcers.
References