Case Report
Multiple Ileal Atresia with Total Colonic Atresia, A Case Report
1Pediatric surgery, Aswan University Hospital, Egypt
2Headmaster of pediatric surgery, Assiut University Hospital, Egypt
*Corresponding author: Sarah Magdy Abdelmohsen, Assistant Lecturer, Pediatric Surgery Department, Aswan University Hospital, Egypt, Tel: +201012069422, E-mail: sosoramily@yahoo.com
Received: June 20, 2017 Accepted: June 24, 2017 Published: June 30, 2017
Citation: Abdelmohsen SM, Osman MA. Multiple Ileal Atresia with total Colonic Atresia, A Case Report. Madridge J Case Rep Stud. 2017; 1(1): 16-19. doi: 10.18689/mjcrs-1000104
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Introduction
Intestinal atresia usually detected in the third trimester of pregnancy with different etiologies report by different authors.
Case report
We had a unique case of multiple ileal atresia, atresia associated with cardiac anomaly and dysmorphic features. Our case had genetic predispositions as she is the 3rd offspring of a consanguineous parent. The 2nd offspring was also a female and died due to neonatal intestinal obstruction.
Histopathological examination of our case showed total colon mucosal atrophy associated with absent ganglion cells and disappearance of myenteric plexus. So, we suggest a presence of association for colonic atresia and absent ganglion cells.
Conclusion Is there a need to modify the usual classification of intestinal atresia?
We recommend that early diagnosis and treatment is important to improve the prognosis in colonic atresia.
Keywords: Multiple ileal atresia; Colonic atresia; Colonic aganglinosis.
Introduction
Atresia—derived from the Greek components a- (“no” or “without”) and tresis (“hole” or “orifice”)—refers to a congenital obstruction with complete occlusion of the intestinal lumen; it accounts for 95% of obstructions [1-5].
Colonic atresia (CA) is a rare cause of congenital bowel obstruction, on average 1 case per year of colonic atresia is being seen in most of the pediatric surgical centers [6]. An incidence (colonic atresia) of 1 in 20,000 lives births and comprise for 1.8−15% of all cases of bowel atresia in newborns [7,9]. The coexistence of colonic atresia and absent ganglia at histopathological examination presents an etiological and diagnostic challenge.
We report a case of multiple ilealatresia with total colonic obstruction associated multiple congenital anomalies for further study.
Case report
A 5-day old female infant, born at ± 36 weeks gestation due to premature rupture of membrane because of polyhydramnios. She delivered by cesarean section, birth weight 2300 gms, AB +ve blood group.
The infant admitted to a neonatal unit at Aswan City, Upper Egypt, at the end of 2ndbirthday due to failure to a passage of meconium and vomiting twice.
The infant parents are relatives, from a rural area of Aswan, Upper Egypt. Her mother is 22 years old, has one normal living son and previous daughter complained of neonatal intestinal obstruction operated at Assiut University Hospital then died at the end of 2ndweek postoperative at Assuit University Neonatal Unit.
No drugs or infection exposure was documented during pregnancy. The mother developed polyhydramnios at the 3rd trimester.
The infant was transferred to our Aswan University Neonatal Unit at the 4thbirthday.
Physical examination showed pale neonate without jaundice or cyanosis. Skin mottling, dysmorphic features (frontal bossing, law set ear, depressed nasal bridge, receding mandible).
Chest examination was clinically free.
Heart examination showed audible ejection systolic murmurs, Echocardiography revealed atrial septal (ASD) 5mm.
Abdominal examination showed marked abdominal distension accompanied by hypoactive intestinal sounds.
The anus was present in its normal position. We tried to introduce small caliber flexible tube into the anus but it's stopped after one and a half cm from the anus, we tried to inject warm saline inside the flexible rectal tube but all saline gone outside the anus. Abdominal and chest x-ray revealed small bowel gaseous distension. The gastrografin enema did not do due to spillage out of all gastrografin solution from the anus.
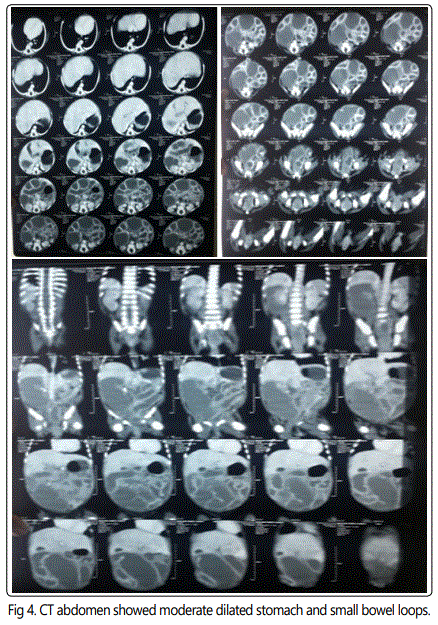
CT abdomen with contrast revealed moderately distended stomach as well as small bowel loops with collapsed ascending, transverse and descending colon.
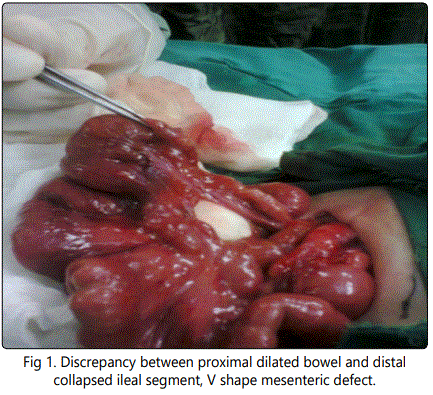
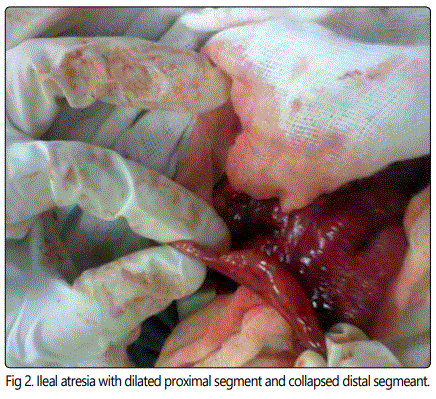
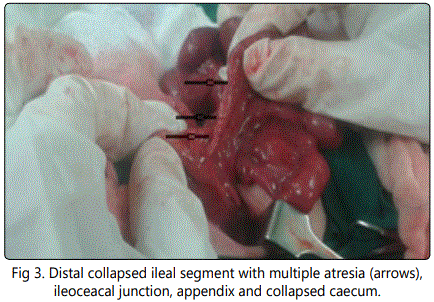
Laparotomy was done by transverse upper abdominal incision extending over both rectus abdominis muscles, there was dilated small bowel loops with multiple ileal atresia started 8-9cm from the ileocaecal junction, the multiple ileal atresia were due to multiple mucosal webs 1-2 cm between each other with intact mural wall associated with V shape mesenteric defect.
Exploration of ascending, transverse, descending colon revealed total colonic atresia by obliterated mucosal lumen all over the whole length of the colon with a just patent rectum and anus.
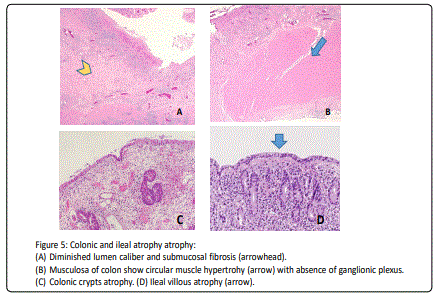
Terminal ileostomy was carried out. The atretic part of ileum and whole obliterated colon were sent for pathological examination which revealed the surface mucosa of the affected ileal segments show marked decrease lumen caliber with uniform villous atrophy. The affected colonic loop exhibits total mucosal atrophy. The submucosa of both ileum segment and colon show extensive fibrosis and congestion with depletion of ganglion cells. The musculosa revealed hypertrophy of circular muscles and disappearance of myenteric plexus. Postoperative the infant started passing from ileostomy at the 2ndpostoperative day, gradual oral feeding started at 4th postoperative day.
At the 10th postoperative day, the general condition of the neonate started to deteriorate with septic shock and apnea developed due to hospital acquired an infection. WBCs increased from 9.000 mcl at times of admission up to 35,000mcl, the infant developed thrombocytopenia, recurrent attacks of hypothermia, hypoglycemia with hemodynamic instability.
Respiratory distress developed with subcostal retractions accompanied by continuing the decrease in oxygen saturation until the neonate shifted from nasal oxygenation to mechanical ventilation.
The infant received 3rd generation cephalosporin, ampicillin, aminoglycoside antibiotic and died at the 12th postoperative day from respiratory failure before blood sample was taken for culture analysis.
Discussion
Intestinal atresia usually detected in the third trimester of pregnancy [1]. The diagnosis of Ileal atresia may be difficult. Polyhydramnios is not routinely present and is more common with jejunal rather than Ileal atresia [10]
Louw et al were believed that the etiological basis of colonic atresia to be a vascular insult to the mesenteric vessels during fetal development [2]. A 2005 study suggests that defects in the fibroblast growth factor 10 (FGF10) pathways may be involved [3].
A report by Benawra et al. [4] of three cases occurring amongst first-degree relatives of a family supports a genetic basis. Here in our case the parents are relatives and had previous female neonate died postoperatively due to intestinal obstruction. Also, our case had multiple congenital anomalies started from dysmorphic features, cardiac anomalies to GIT anomalies. Also, we support the vascular insult theory as our case had a defect of mesentery at the ileal segment atresia and the question is the genetic predispositions have a role in intrauterine vascular insults and intestinal atresia?
Multiple atresias have been described in infant of mothers with graft-versus-host disease and immunosuppression and those with malformations processes that are likely due to autosomal recessive transmission [11,12]. which supports the theory of genetic basis.
Colonic atresia has been associated with numerous anomalies, including omphalocele, gastroschisis, exomphalos, vesicointestinal fistula, Hirschsprung's disease and imperforate anus, as well as choledochal cyst [13-17].
In our case, the whole colon was atretic with mucosal atrophy but with intact mural wall associated with absent ganglion cells and disappearance of myenteric plexus, does there any relation between absent ganglion cells and colonic atresia? Further studies need for an answer this question?
Grosfeld et al. classification of intestinal atresia, which is currently the most commonly used classification scheme [18]:
At our case, the classification is a complex between type I and type IV as the ilealatresia was multiple associated with a defect in mesentery but with an intact mural wall, also it associated with total colonic atresia, so, we think that we in need to a modified specific classification for multiple intestinal atresia.
The delay in diagnosis of colonic atresia beyond 4 days may result in mortality as high as 100% (19). Our case was transferred late to Aswan University Neonatal Unite due to a limited number of our incubators. Finally, our case is one of the rare cases of multiple intestinal atresia with complete total colonic lumen occlusion associated with the disappearance of myenteric plexus.
Conclusion
Is there a need to modify the usual classification of intestinal atresia? Is there an association for colonic atresia with absent ganglion cells and disappearance of myenteric plexus? We need more advanced research about this association. Also, we believe in genetic association for intestinal atresia and needs more study about the responsible gene mutation. We recommend that early diagnosis and treatment is important to improve the prognosis in colonic atresia.
Acknowledgement
To Dr. Marwa T. Hussien pathologist at South Egypt Cancer Institute, Assiut University, Assiut, Egypt for her microscopic photo picture.
References