Case Report
Alar Rim Reconstruction: A Case Report and Review of the Literature
Department of Surgery, Division of Plastic and Reconstructive Surgery, Rutgers New Jersey Medical School, Newark, NJ, USA Running head: Alar Rim Reconstruction
*Corresponding author: Ramazi O Datiashvili, Division of Plastic and Reconstructive Surgery, Department of Surgery, Rutgers New Jersey Medical School, Newark, USA, Tel: (973) 972-5377, Fax: (973) 972-8268, E-mail: datiasro@rutgers.edu
Received: September 4, 2016 Accepted: June 22, 2017 Published: June 29, 2017
Citation: Therattil PJ, Kass WS, Sood A, Datiashvili RO. Alar Rim Reconstruction: A Case Report and Review of the Literature. Madridge J Case Rep Stud. 2017; 1(1): 11-15. doi: 10.18689/mjcrs-1000103
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Reconstruction of alar rim and soft triangle defects provide a unique challenge to plastic surgeons. As with most areas that are difficult to recreate, there are many reconstructive options, but none consideredto be the gold standard.
Methods: A literature review was performed using Medline and the Cochrane Collaboration Library for primary research articles on alar rim reconstruction. Data related to surgical techniques and outcomes were extracted. In addition, the authors describe their experience using a chondrocutaneous composite graft from the helical root to reconstruct an alar rim defect.
Results: The literature search yielded 63 relevant studies that met inclusion criteria. Composite grafts are a well-accepted reconstructive option for defects less than 1.5 cm. Adjuncts such as post-operative cooling, hyperbaric oxygen, cartilaginous perforations, extended dermal pedicles, decongestion, platelet-rich plasma or stem cell therapy may increase graft survival, or may allow for grafts larger than 1.5 cm to survive. For larger defects, transposition flaps or forehead flaps may be needed. If a transposition flap is used, the flap choice and its design are important in preventing complications.
Conclusion: There are many options for reconstruction of small alar rim and soft triangle defects. Composite grafts provide an excellent option to reconstruct skin, cartilage, and lining in a single stage. When composite graft size is restricted and post-operative cooling is implemented, graft survival appears to be high. Other adjuncts exist to enhance graft survival, but are limited by access, cost, or complexity of application.
Keywords: Alar rim; Auricular graft; Chondrocutaneous graft; Composite graft; Nasal ala; Plastic surgery; Soft triangle; Reconstruction.
Background
Minor deformities of the nose from acquired facial trauma or oncologic resection may not necessarily impair the functions of olfaction or speech, but may affect respiration and may alter how an individual is perceived by society [1]. Therefore, it is critical, even in reconstruction of small defects of the nose, to optimize the aesthetic outcome.
Reconstruction of the nose is particularly demanding because of its variable skin texture, contour, and color. Recreating the curvatures and subtle shadowing of nasal features while predicting scar contracture is a delicate balance. Certain tenets should be followed when attempted to recreate the idiosyncrasies of the nose. One should consider the aesthetic subunits: the dorsum, sidewalls, tip, soft triangles, columella, and alae. If a defect involves more than 50% of a subunit, one should consider excising the remainder of the subunit and reconstructing it as a whole. In this way, the edges of the reconstructed area are blended with the natural ridges and valleys of the nose. The undamaged contralateral subunit is used as a template for reconstruction [2]. The location and size of the injury as well as the layers involved will determine the appropriate reconstruction.
Reconstruction of the alar rim and soft triangle is especially difficult. The anatomy of the region is deceptive; the lower lateral cartilage does not actually lie directly at the border of the alar rim, but instead lies more superiorly with the skin hanging down and supported by fibro fatty attachments [3]. When these attachments are lost, they cannot be recreated, which necessitates the addition of cartilaginous support at the border of the alar rim where there was none initially. Without cartilaginous support, the external nasal valve will collapse, resulting in difficulty breathing [4]. As with most regions that are difficult to recreate, there are many possible options for alar rim reconstruction with none of them being considered the gold standard. Because of the myriad of options, we discuss the available literature on reconstruction of the alar rim and soft triangleand describe our reconstruction of choice.
Methods
Medline and Cochrane databases were thoroughly searched by the authors from January 1975 through July 2016. In addition, bibliographies of each relevant citation were reviewed for additional sources. The following search terms were used as both subjects and key words: ‘reconstruction’ And (‘alar rim’ or ‘soft triangle’).
The initial PubMed search yielded 247 studies. The Cochrane database search yielded 1 study. Two independent reviewers evaluated the titles and abstracts of all studies without language restrictions and subsequently included or excluded studies based on the inclusion and exclusion criteria.
The authors included studies that were published in scientific journals and involved patients who underwentalar rim or soft triangle reconstruction. The authors excluded studies that were focused on procedures unrelated to alar rim reconstruction, or those that focused on restoring alar rim contour after rhinoplasty with no soft tissue defect.Ultimately, 63 studies met the inclusion criteria. Manuscriptsof these studies were reviewed as a second stage to examine the surgical indication, method of reconstruction, and outcome, which are reviewed in the discussion below.
Below the authorsalso describe the case of a patient who underwent reconstruction of a full-thickness defect of the alar rim and soft triangle after a traumatic avulsion during a bicycle accident.
Case Report
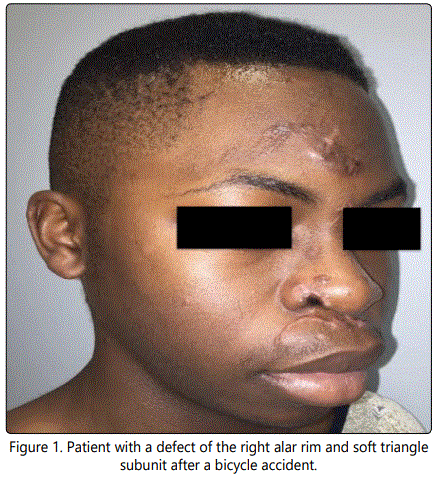
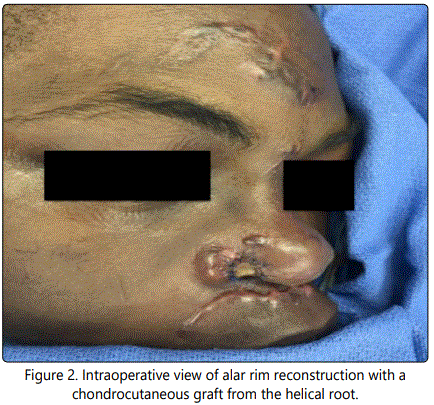
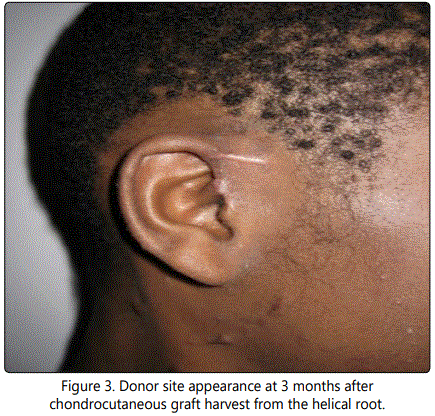
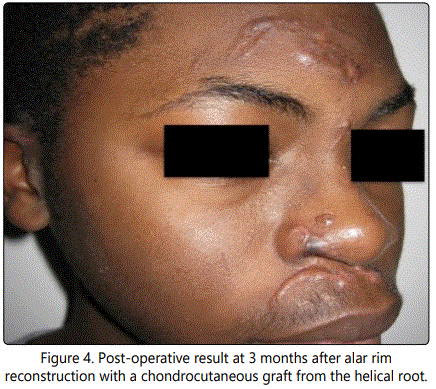
A 14 year-old male presented to the emergency room after being struck by a car while riding his bicycle. The patient was ejected from the bicycle, landing face first onto the pavement. The patient had multiple facial lacerations, including a full-thickness defect of the ala and soft triangle aesthetic subunits of approximately 1.3 cm (Figure 1). The patient had repair of his facial lacerations, except for the defect of the nasal ala; this was initially allowed to undergo secondary intention with plans for delayed reconstruction. Ultimately achondrocutaneous graft from the ipsilateral helical root was utilized for reconstruction (Figure 2). Because the defect affected less than 50% of the ala, we elected not to resect the remainder of the subunit. The wound edges were refreshed and the composite graft was trimmed to fit the defect with a small amount of excess bulk to account for contraction. The graft was inset with interrupted, deep 5-0 Monocryl(Ethicon Inc., Somerville, NJ) sutures to stabilize the cartilage and simple, interrupted 5-0 Prolene(Ethicon Inc., Somerville, NJ) sutures for exact skin approximation. The donor site was closed with a Dieffenbach “winged V-plasty,” which healed without any complication (Figure 3) [5]. Post-operative cooling of the graft was initiated immediately after surgery, with ice packs being applied every 2 hours (for 15 minutes at a time) for 24 hours. Postoperatively, there were no complications; the graft completely survived, and both the donor and the recipient sites healed well (Figure 4). The patient and his parents were satisfied with the aesthetic appearance despite some degree of color mismatch. He did not have any respiratory issues or external valve collapse on inspiration.
Results and Discussion
“Low rungs” of the reconstructive ladder
For very small defects, primary closure gives the best aesthetic outcomes, but rarely is possible. More often, primary closure causes excessive distortion of the nasal ala and eliminates any possibility of symmetry.Secondary intention for defects at the alar rim usually results in poor contour, notching, and valvular dysfunctionin addition to time and inconvenience associated with dressing changes.
When dealing specifically with alar rim defects, a skin graft may be appropriate from a color-matched donor site if the defect is superficial, involving only skin and subcutaneous tissue, and is less than 1 cm [6]. However, the contour, color, and texture match are usually poor, and some degree of alar notching is likely. Alar cartilage is usually a poorly vascularized bed for graft acceptance, and thus there is a high risk of partial necrosis of the graft and wound contracture resulting in valvular dysfunction [7]. If perichondrium is not intact, nasalis or columellar turnover flaps can be utilized to create a wellvascularized bed for grafting [8]. The use of perichondrial cutaneous grafts, which includes an underlying layer of perichondrium and its associated vascular plexus, has been supported by some authors because of its potential for better graft take with less contraction and notching [9,10].
Composite chondrocutaneous grafts
Achondrocutaneous composite graft can be used reliably for full-thickness defectsof the alar rim up to 1.5 cm. The graft will retract naturally over time and thus designing a graft that is 10-20% larger than the defect will give a more natural result [11]. Donor sites for a chondrocutaneous graft are typically selected from the patient's ears, usually from the helical root, helical rim, conchal bowl, or antitragus [5,12]. A composite chondrocutaneous graft is initially only supplied by plasma imbibition, in which it absorbs nutrients by passive diffusion. Inosculation occurs after 3 days, in which the vessels at the base of the graft form connections with those at the base of the wound. Angiogenesis occurs after approximately 5 days, with new blood vessels growing into the graft.
There are several tenets to optimize composite graft survival that are perpetuated, some of which are only weakly supported by evidence-based literature. Because of the tenuous nutrient supply and the higher metabolic demand of composite grafts, the metabolic needs of the graft must but reduced immediately after grafting [4]. Metabolic demand can be reduced by cooling it with ice packs (every 2 hours for 15-20 minutes at a time in one series) [13]. The optimal temperature and duration of cooling have not yet been determined. Grafts as large as 2-3 cm have been reported to survive reliably with postoperative cooling to 5-10°C [14,15]. Some authors suggest limiting graft size such that no portion of the graft is more than 0.5 cm from the vascularized bed [4]. Chandawarkar et al. (2003) described a modified composite graft with an extended dermal pedicle. The dermal pedicle is buried in a soft tissue pocket at the recipient site to increase the surface area by which the graft can receive diffused nutrients [15]. The cartilage can also be perforated to increase nutrient diffusion to the central portions of the graft that are most prone to necrosis [16]. Venous congestion of composite grafts should be expected and thus, some authors advocate for scarification of the graft and topical Heparin solution to aid in decongestion [17].
Patient selection is also critical to optimize graft survival. Patients at risk for poor graft take include smokers, diabetics, and those with peripheral arterial disease.(4) The addition of hyperbaric oxygen may improve the survival of larger composite chondrocutaneous grafts by enhancing fibroblast replication and neovascularization [18,19]. Platelet richplasma (PRP) and adipose-derived mesenchymal stem cells have also been shown to increase graft survivability in animal models [20,21].
Another consideration is providing adequate support to the alar rim. A typical composite chondrocutaneous graft only provides a small button of cartilage within the graft. Alternatively, the composite graft can be fashioned with cartilaginous pegs extending from the main portion of the graft in order to help span the defect completely [22,23]. In addition, instead of a true composite graft, one can fabricate a composite graft from a strip of cartilage and an overlying skin graft.
Complications of composite grafting include necrosis, poor graft appearance, infection, or displacement. Any eschar that develops from necrosis should be left alone as the necrosis may only be partial thickness and the eschar provides a biologic barrier from infection [4]. Patients should be warned about the progression of graft appearance, as the final skin color may not be present until 1 to 2 years after surgery. Skin color mismatch can be problematic in the long-term, especially in darker skin types.
Flap reconstruction
For larger defectsof the alar rim and soft triangle (usually those greater than 1.5 cm), skin grafts or composite grafts may not be adequate. Local flaps usually provide a better texture and contour with the expense of additional scars. The bilobed flap (Zitelli-modification) is a workhorse for nasal reconstruction and can be used for alar reconstruction. There are good reasons, however, as to why bilobed flap do not result in good outcomes when used for very distal nasal defects. The oblique orientation of the second lobe of the flap can result in elevation of the contralateral ala. In addition, when the defect is at the most distal portion of the nose, the skin being transposed is from immobile skin of the nasal tip, putting the closure under undue tension. Transposition flaps also have a Z-plasty lengthening effect that is more pronounced when the individual flaps undergo larger degrees of rotation. As a result, bilobed flaps have a “bulldozing” effect and displace the ipsilateral ala in a downward direction. A trilobed flap can be used to reorient the tension vector to be more vertical, borrowing tissue from the supple skin of the nasal sidewall and preventing elevation of the contralateral ala.It also prevents “bulldozing” of the ipsilateral ala by reducing the degree of rotation of each lobe [7].
A number of variations of nasolabial flaps can be used, usually superiorly-based. These nasolabial flaps can be kept relatively thin, as they are axial flaps based on branches of the facial, infraorbital, and transverse facial arteries. It is important to preserve the alar-facial sulcus during nasolabial flap planning, if possible, as it is difficult to recreate [24]. The inferiorly-based paranasal or nasal dorsum flaps are other possible donor sites [8,25]. The use of the above flaps for small defects, however, usually requires multiple stages. Alternatively, a medially-based “hinge” turnover nasolabial flap can be used for a single-stage reconstruction [26].
For defects greater than 2.5 cm in diameter, distant flap transfer from the forehead is needed [3]. While pedicled flaps can reconstruct very large, even near-total, defects of the nose, the donor defects may be much more obvious and the delicate contours of the nose may be obliterated. Often multiple secondary procedures, including debulking, are needed to achieve an acceptable result. It is important to note that even when nasolabial or forehead flaps are brought in, there is still a very high risk of alar notching (up to 46-65% in one series) [11]. The main limitation of composite grafts is size of the defect. Some authors, combining the advantages of composite grafts and flaps, have performed alar reconstruction with composite free flaps from the helical root. The major limitation of free flaps from the helix is the short pedicle length, which some surgeons have addressed by using vein grafts or reverse flow flaps based on the parietal and frontal branches of the superficial temporal artery [27].
Constantine et al. (2013) aptlysummarize reconstruction of alar rim/soft triangle defects depending on the layers involved. In Type I defects, the skin was intact, but with cartilaginous and mucosal lining defects. In these cases, a composite graft from the conchal bowl was used to provide support and lining. In Type II defects, the mucosa was intact with an absence of cartilage and overlying skin. A nasolabial flap with an underlying cartilage graft was used in these cases. In Type III defects, all three tissue layers were affected and a paramedian forehead flap was used in conjunction with a cartilage graft to reconstruct all three layers [28]. An argument could be made that all of these defects could be reconstructed with composite chondrocutaneous grafts when defects are less than 1.5 cm.
Alternative methods of reconstruction
In patients with flaring alae, such as Asian patients with a low, flat nose, correction may even be achieved with primary closure of the nasal alar rim and contralateral alar reduction. If the patient has a particularly bulbous tip, another option is bilateral alar cartilage reduction rhinoplasty [29]. In this technique, flaps of skin, subcutaneous tissue and muscle are elevated just above the perichondrium. Incisions are kept within the aesthetic borders of the nose. The alar cartilages are trimmed and interdomal sutures are placed to reduce the volume of the tip and to create soft tissue redundancy forprimary closure. The same technique can be used, but with a Z-plasty for the closure [30]. Alternatively, a full-thickness Z-plasty can be performed as described by Denonvilliers [31].
Conclusions
There are many options for reconstruction for alar rim and soft triangle defects, especially small defects (less than 1.5 cm). We believe that composite chondrocutaneous grafts provide an excellent option to reconstruct skin, cartilage, and lining with a good cosmetic result in a single stage. When composite graft size is restricted and post-operative cooling is implemented, graft survival appears to be high. Other adjuncts exist to enhance graft survival (hyperbaric oxygen, cartilaginous perforations, extended dermal pedicles, decongestion, PRP or stem cell therapy), but are limited by access, cost, or simplicity of application.
References