Research Article
Validation of the Endothelial Markers Cd31 and Cd34 in Immunohistochemistry of the long Saphenous Vein for Coronary Artery Bypass Surgery
1Department of Cardiothoracic Surgery, University Hospital of South Manchester NHS Foundation Trust, Manchester, UK
2The Transplant Centre, University Hospital of South Manchester NHS Foundation Trust, Manchester, UK
3Department of Histopathology, University Hospital of South Manchester NHS Foundation Trust, Manchester, UK
4School of Nursing and Midwifery, The University of Manchester, Manchester, UK
*Corresponding author: Bhuvaneswari Krishnamoorthy, (current NIHR clinical Research fellow), Lead Surgical Care Practitioner, Cardiothoracic surgery, University Hospital of South Manchester, NHS Foundation Trust, Manchester, UK, Tel: 0044 161 291 2078, Fax: 0044 161 291 5024, E-mail: bhuvaneswari.bibleraaj@uhsm.nhs.uk
Received: October 19, 2016 Accepted: November 11, 2016 Published: November 15, 2016
Citation: Krishnamoorthy B, Critchley WR, Bishop P, et al. Validation of the Endothelial Markers CD31 and CD34 in Immunohistochemistry of the long Saphenous Vein for Coronary Artery Bypass Surgery. Madridge J Cardiol. 2016: 1(1): 8-11. doi: 10.18689/mjc-1000103
Copyright: © 2016 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objectives: Endothelial injury during a surgical intervention can significantly affect the functional status of the vein. The endothelial layer plays a vital role in the long saphenous vein for ensuring smooth blood flow and the prevention of vasoconstriction and thrombi formation within the blood vessels. There are few histological studies comparing the different vein harvesting techniques that have studied endothelial layer integrity using CD31 and CD34 on human long saphenous veins.
Methods: Non-distended vein samples measuring 1cm were obtained from ten consecutive traditional open vein harvesting patients and were automatically processed and stained using immunohistochemistry for CD31 and CD34. The colour, intensity and distribution of the staining on the tissues was scored blindly by five independent scientists and an expert histopathologist for this study.
Results: The CD34 antibody demonstrated greater colour (p<0.007), intensity (p<0.019) and distribution (p<0.007) compared to CD31.
Conclusion: Our study indicates that CD34 provides a more reliable endothelial marker in the long saphenous vein than CD31. The results of this study can be translate into other immunohistochemistry studies looking at the quality of the endothelium on the vein in cardiac and vascular surgical studies.
Keywords: Coronary artery bypass surgery; Endothelium, immunohistochemistry; Blood vessels.
Introduction
Coronary artery bypass surgery (CABG) is the most commonly performed procedure in cardiac surgery. This method involves bypassing blocked coronary arteries using arterial and venous conduits. Despite the use of arterial conduits providing improved long term graft patency, venous conduits such as the long saphenous vein are still widely used in multiple bypass surgery due to its long length and easy availability. However, donor leg wound complications are among the most common post-surgical problems, which may occur in 5% to 44% of cases [1]. Minimally invasive vein harvesting methods have been developed to reduce the risk of wound complications and post-operative morbidity. However, these techniques have been associated with greater risk of damaging the vein layers, particularly of disrupting the endothelium, during surgery. If this occurs, platelets become aggregated and induce endothelial denudation, promoting intimal proliferation and hyperplasia, leading to graft occlusion, which may subsequently result in poor long term graft patency [2].
Previous studies have focused on endothelial damage occurring during harvesting of the long saphenous vein, predominantly via assessment of CD31 expression. CD31 is a 130-kDa transmembrane glycoprotein, which demonstrates strong homogeneous expression in all human pulmonary endothelial cells but is also expressed to a lesser extent on platelets and some leukocyte subsets [3]. CD34 has also been used as a marker of endothelial cells. However, a systematic comparison of the quality of these markers would be beneficial, especially considering that the molecular and functional characteristics of endothelial cells can vary on the vascular tree between the different vessels around the body [4]. The 110-kDa transmembrane glycoprotein CD34 demonstrates a more heterogeneous expression and is particularly found on endothelial cells of: capillaries, arteries, veins, arterioles and venules [5]. Additional markers expressed on endothelial cells, such as von Willebrand Factor (vWF) and Fli1, have been utilised previously for the identification and detection of these cells. The glycoprotein vWF has important roles in platelet adhesion following injury, and is expressed on endothelial cells in a range of settings. However, vWF has been previously demonstrated to have weak expression on capillary endothelium and its use may be complicated subendothelial expression in certain tissues. Fli1 is consistently expressed by endothelial cells in a range of tissues, however it is also present within the nucleus of haematopoietic cells, especially lymphocytes and is a useful marker for diagnostic evaluation and detection of vascular tumours. This study evaluated only the use of CD31 and CD34 for assessment of endothelial integrity on the long saphenous vein due to the nature of their expression patterns.
Immunohistochemistry (IHC) remains the gold standard for studying the morphological status of the vein. Although previous studies have used IHC to score endothelial integrity, none have directly compared the quality of CD31 or CD34 staining following vein harvesting for CABG surgery. This is the first study to compare the difference in colour, intensity and distribution of CD31 and CD34 expression on long saphenous vein sections with the purpose of identifying a standard marker for future IHC for use in assessing endothelial integrity.
Methods
Sample collection
An overview of the experimental design for this study is included in figure 1. Ethical approval was provided by the Greater Manchester North East - National Research Ethics Committee (NREC) as part of the vein integrity and clinical outcomes (VICO) randomised controlled trial. The VICO trial is designed to assess the direct relationship between endothelial damage and clinical outcomes. Samples of proximal long saphenous veins were collected from the lower leg using the open vein harvesting technique from ten consecutive patients. Vein conduits retrieved by minimally invasive vein harvesting techniques were not included in this study to ensure a reliable sample was retrieved with intact endothelium. Vein samples that were not surgically distended were utilised in this study to provide a reliable result indicative of viable endothelium in the long saphenous vein. Samples were cut into approximately 1cm sections and placed into a solution of 4% formalin in distilled water (pH 7.4). Samples were processed using the standard operating procedures of the Histopathology Laboratory at UHSM. Briefly, formalin fixed samples were paraffin embedded, then cut into 4 µm-thin sections, dewaxed and rehydrated in graded alcohols. Endogenous peroxidase activity was quenched by incubation in a 0.3% hydrogen peroxide aqueous solution for 15 minutes at room temperature. The heat-induced epitope retrieval method by means of pressure cooker was used for antigen retrieval of vein cross sections.
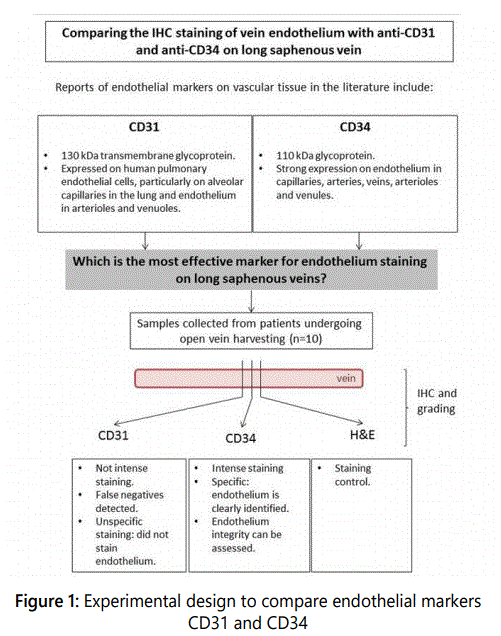
The efficacy of anti-CD31 and anti-CD34 antibodies (Dako, Cambridgeshire, UK) to stain the saphenous vein endothelium was compared following automated tissue immunohistochemistry using both antibodies at a 1:30 dilution in DAKOTM antibody diluent (Dako, Cambridgeshire, UK) according to manufacturerʼs protocol. Detection was performed using the ImmPress HRP universal antibody polymer detection kit (Vector Laboratories, UK) and the ImmPact DAB peroxidase (HRP) substrate (Vector Laboratories, UK) according to manufacturerʼs protocol. Haematoxylin and eosin staining was performed for the evaluation of the saphenous vein, as a method for counterstaining (see figure 2).
Each slide was allocated a random number before any assessors assigned a score. The slides were imaged using Pannoramic 250TM slide scanner at The University of Manchester. This machine has a special high-NA Carl ZeissTM optic lens to achieve maximum resolution of up to 0.16 µm per pixel image. Samples were scored by five blinded, independent and fully trained assessors by using Pannoramic ViewerTM software for efficient image viewing, annotation and archiving purposes. All the scores were verified by a UHSM Consultant Histopathologist. None of these assessors were involved at any stage of this research project. The slides were assessed for endothelial integrity (inter assessor variability was <15%). Slides were scored based on the colour, intensity and staining distribution of CD31 and CD34 using the following validated scoring system: “1” neg-none, “2/+” mild, “3/++” moderate and “4/+++” intense [4, 6].
Statistical analysis
All data was expressed as percentages, with differences between the two sets of results determined using the Chisquare test for categorical variables. A p-value of <0.05 was considered statistically significant. SPSS 19.0 software was used for all calculations.
Results
Consecutive saphenous vein sections were stained using anti-CD31 and anti-CD34 antibodies. A significantly different pattern of expression was found in terms of colour, intensity and distribution as follows:
Colour
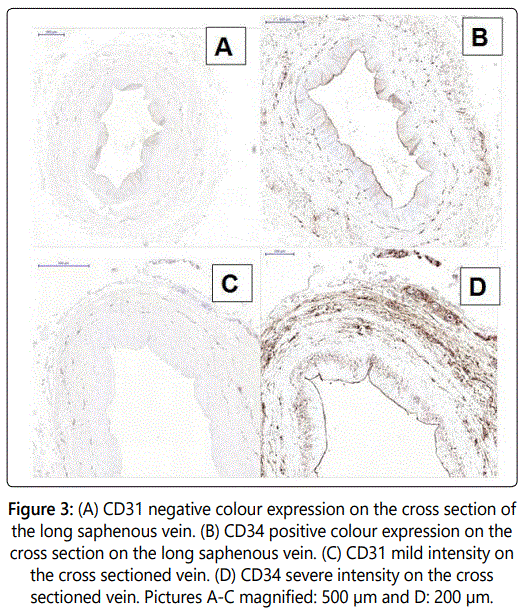
The relative colour of CD34 expression on veins was found to be more distinct than that of CD31 (80% negligible and 20% mild colour vs. 60% moderate and 40% strong colour distinctiveness for CD34, p=0.019, see figures 3a and 3b).
Intensity
Endothelial cell staining was found to be significantly more intense with the use of anti-CD34 antibody, compared to the mild staining of CD31 (100% mild staining for CD31 vs. 20% mild, 40% moderate and 20% strong staining for CD34.
Distribution
staining was more widely distributed across the tissue compared to CD31, with improved coverage of endothelial cells (100% negligible staining for CD31 vs. 40% mild and 60% moderate distribution of the stain for CD34, p=0.007). The CD34 stain was uniformly distributed along the endothelial layer of the saphenous vein. In addition, small capillary vessels on the adventitial layer were also effectively stained. In contrast, CD31 staining using anti-CD31 was found to be irregular along the endothelial layer.
Discussion
This study aims to compare the use of CD31 and CD34 as markers of endothelium on human long saphenous vein. Our findings indicate that CD34 stains in a more intense and regular manner, including the endothelium of small arterioles and venules located in the tunica intima, compared to CD31. Modern bioimaging techniques represent a fundamental area for evaluation of tissue samples at a cellular level, yet highly optimised staining is required for reliable scoring. This is particularly true for large scale studies when significant numbers of samples need to be assessed as high throughput automated methods can be utilised where bright and distinct staining is present.
The majority of studies assessing endothelial integrity have employed CD31 as the key marker, which is expressed on ~90% of endothelial tumours [7], ~90% of vascular tumours and sinusoids of the spleen [8]. CD31 is also strongly expressed on the surface of circulating platelets, monocytes, neutrophils and intracellular junctions, making interpretation difficult [3]. Its frequent use in analogous studies, without evidence of systematic comparison with other markers, led to its acceptance as the best single marker for this purpose. In contrast, CD34 is assumed to play a major role in the formation of endothelial adherence junctions, which are the key components of angiogenesis [9,10]. It is also present on lympho-haematopoietic stem and progenitor cells, leukemic cells and embryonic fibroblasts. In routine clinical practice, this marker is used for leukaemia diagnosis using immunohistochemistry and for the purification of immunological stem cells for clinical transplantation [11,12]. Most of these studies focused on comparing these markers on a macro rather than a microvascular level, which could pose important biological and physiological differences. In addition, there is limited evidence comparing CD31 and CD34 in human long saphenous vein.
Further knowledge regarding the expression pattern of specific endothelial phenotypes on the vascular tree is important to evaluate the effectiveness of these markers. Although previous studies did not perform a comparison between CD31 and CD34 in human long saphenous vein, the results of our study suggest that CD34 is a superior marker to CD31 in determining the presence of endothelial cells on the vessel luminal wall.
In conclusion, the use of CD34 provides a stronger and more distinct staining pattern for endothelium in human long saphenous vein samples when compared to CD31. This study provides novel evidence regarding the use of these markers which can have important clinical utility, such as when used as indicators of endothelial denudation following harvesting for coronary artery bypass surgery.
On behalf of all authors, I can confirm that this manuscript has not been submitted or previously published elsewhere and all authors have read and approved the final manuscript. Furthermore, there is no conflict of interest to declare in association with this manuscript.
References
- Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002; 72(3): 221-229. doi: 10.1006/exmp.2002.2424
- Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006; 54(4): 385-395. doi: 10.1369/jhc.4A6514.2005
- Nezafati MH, Nezafati P, Amoueian S, Attaranzadeh A, Rahimi H R. Immunohistochemistry comparing endoscopic vein harvesting vs. open vein harvesting on saphenous vein endothelium. J Cardiothorac Surg. 2014; 9: 101. doi: 10.1186/1749-8090-9-101
- Tanigawa N, Lu C, Mitsui T, Miura S. Quantitation of sinusoid-like vessels in hepatocellular carcinoma: its clinical and prognostic significance. Hepatology. 1997; 26(5): 1216-1223. doi: 10.1053/jhep.1997.v26.pm0009362365
- Sauter B, Foedinger D, Sterniczky B, Wolff K, Rappersberger K. Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells. Differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem. 1998; 46(2): 165-176.


