Review Article
Advances in the Management of Non-Small Cell Lung Cancer (NSCLC)
R&D Department, Cadila Pharmaceuticals Limited, Ahmedabad, India
*Corresponding author: Bakulesh Khamar, R&D Department, Cadila Pharmaceuticals Limited, Ahmedabad, 382210, India, Fax: 0091-2718-225031, Tel: 0091-2718-225001, E-mail: bmk@cadilapharma.co.in
Received: July 30, 2018 Accepted: September 25, 2018 Published: September 30, 2018
Citation: Khamar B. Advances in the Management of Non-Small Cell Lung Cancer (NSCLC). Madridge J Cancer Stud Res. 2018; 3(1): 78-84. doi: 10.18689/mjcsr-1000112
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Recent findings from studies [KEYNOTE 10, 24, 189, 407 (pembrolizumab); Check Mate-17,57,227 (nivolumab); IM power 131,150,OAK (atezolizumab)] using checkpoint inhibitors as a monotherapy as well as in combination of chemotherapy has demonstrated improved outcome in patients with advanced NSCLC without actionable mutation driver and also showed a tolerable toxicity profile and durable response. Based on analysis of studies performed in the first line management of advanced NSCLC, pembrolizumab is preferred for patients without actionable driver mutation. Pembrolizumab should be used as a monotherapy in patients with PD-L1 expression ≥ 50%. In others, it should be added to chemotherapy. For patients with actionable driver mutation, osimertinib for sensitizing EGFR mutation is preferred over afatinib, gefitinib, erlotinib as a first line therapy. For patients with ALK rearrangement alectinib is preferred over crizotinib restricting use of crizotinib as first line therapy to patients with ROS1 rearrangement. Dabrafenib + trametinib have been found effective in patients with BRAFV600E mutations.
Keywords: Check point inhibitors, Pembrolizumab, Nivolumab, Atezolizumab, Durvalumab, Osimertinib, Crizotinib, Alectinib, Trametinib, Dabrafenib, NSCLC, driver mutations, PD-L1, mutation burden.
Introduction
Lung cancer is one of the major cancers diagnosed worldwide and accounts for nearly 20% of all cancer-related deaths [1]. Non-small cell lung cancer (NSCLC) accounts for about 85% of all primary lung cancers. At the time of diagnosis most of the patients have advanced disease and are not amenable to curative treatment [1-2]. Traditionally NSCLC is treated with cytotoxic chemotherapy based on histological diagnosis. Targeted therapies for NSCLC [3-5] have changed this. They are better tolerated and provide significantly higher response rate. Targeted therapy is indicated in patients having specific oncogenic driver alterations [6]. A prior confirmation of mutation by diagnostic test guides initiation of appropriate targeted therapy. Checkpoint inhibitors are latest in the list of immunotherapy approved for NSCLC [5]. They have changed the outcome by providing durable responses with manageable toxicities. They are used as first line, second line and maintenance therapy as a monotherapy as well as in a combination with chemotherapy. Evaluation for PD-L1 expression level and mutation burden are new diagnostic tests which are helpful in identifying patients who are likely to respond better to checkpoint inhibitors [5]. Of all the checkpoint inhibitors, nivolumab, pembrolizumab, atezolizumab and durvalumab are more advanced and approved in the management of NSCLC [5]. They were originally approved for second line therapy but newer data has established their role in the first line therapy, also. This has resulted in significant changes in the way advanced NSCLC needs to be managed. Checkpoint inhibitors have not been found useful in the management of patients with EGFR mutations, ROS1rearrangements, ALK rearrangements and BRAF V600E mutations. Novel agents are also approved in management of this group of patients. In this article recent developments in the management of advanced NSCLC are reviewed in two sections:
Checkpoint Inhibitors
Checkpoint inhibitors as a First Line Therapy
As a first line therapy checkpoint inhibitors are useful as a monotherapy as well as in combination with chemotherapy in patients with negative or unknown tests results for EGFR mutations, BRAF V600E mutations, ALK rearrangements, and ROS1rearrangements.
Monotherapy
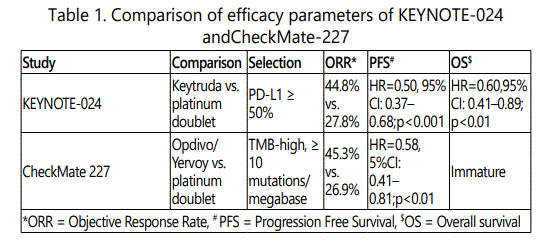
Pembrolizumab (KEYNOTE-10) as well as Nivolumab+Ipilimumab (CheckMate-227) have been evaluated successfully as a sole therapy in management of advanced NSCLC [Table-1].
Pembrolizumab
Pembrolizumab was evaluated as a monotherapy in the KEYNOTE-024 study in first line setting in patients with NSCLC expressing PD-L1 ≥ 50%. 305 patients were randomized to receive pembrolizumab (200 mg fixed dose every 3 weeks) or investigator's choice of platinum-based chemotherapy. Patients eligible for EGFR inhibitors or ALK inhibitors were excluded. Patients receiving pembrolizumab showed improved response to therapy [Objective Response Rate (ORR) 44.8% s. 27.8%]. This was associated with improvement in median Progression Free Survival (PFS) by 4.3 months (median 10.3 vs. 6.0 months; Hazard Ratio (HR) 0.5, 95% Confidence Interval (CI): 0.37-0.68, p < 0.001) and Overall Survival (OS) at 6 months compared with chemotherapy [80.2% vs. 72.4% (HR 0.6, 95% CI: 0.41–0.89, p = 0.005) [7].
Nivolumab+Ipilimumab
Combination of checkpoint inhibitors (Nivolumab+ Ipilimumab) was evaluated against chemotherapy in patients with advanced NSCLC (squamous and non-squamous) in the Check Mate 227 study in first line setting. All patients had high tumor mutational burden [more than 10]. Nivolumab+Ipilimumab increased response rate by 18.4% (45.3% vs. 26.9%) as well as improved PFS (HR 0.58; 95%CI: 0.41– 0.81; p<0.001) compared to chemotherapy, regardless of tumor PD-L1 expression. Survival data is immature [8].
Combination with Chemotherapy
[Table-2] Check point inhibitors have been evaluated with chemotherapy in management of squamous as well as non-squamous NSCLC.
Squamous NSCLC
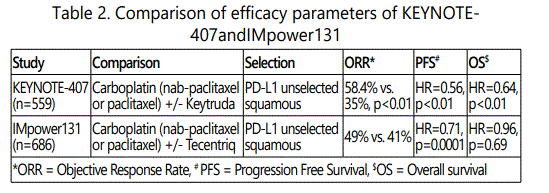
Pembrolizumab [KEYNOTE-407] as well as atezolizumab [IM power 131] has been evaluated in combination with paclitaxel containing platinum doublet [Table-2]. Both are associated with improved outcome.
Pembrolizumab
Pembrolizumab in combination with carboplatin + nab-paclitaxel/paclitaxel as first line treatment was evaluated in the KEYNOTE-407 clinical study in patients with advanced, squamous cell NSCLC. Combination was associated with increase in response rate by 23.4% [ORR 58.4% vs. 35%, p<0.01]. Improved response rate was also associated with improved PFS [HR=0.56, p<0.01] and improved OS (HR=0.64, 95% CI: 0.49-0.85, p=0.0008) at a median follow-up of 7.8 months in patients who were treated with combination pembrolizumab + chemotherapy compared to patients treated with chemotherapy alone. Additionally, OS benefit was seen irrespective of tumor PD-L1 status (Tumor performance Score (TPS)<1%, HR=0.61 [95% CI: 0.35-0.98]; TPS 1–49%, HR=0.57 [95% CI: 0.36–0.90]; TPS>50%, HR=0.64 [95% CI: 0.37–1.10]). Grade 3-5 Adverse Events (AEs) were comparable across the pembrolizumab/chemotherapy and placebo cohorts (69.8% vs. 68.2%, respectively) [9].
Atezolizumab
The IM power 131 study evaluatedatezolizumab + carboplatin & nab-paclitaxel/paclitaxelin patients with advanced squamous NSCLC. Addition of atezolizumab improved response rate by 8% (49% vs 41%). Improvement in PFS was 0.7 months (6.3 months vs 5.6 months, HR=0.71 [95% CI: 0.60–0.85], p <0.0001). Improvement in OS was of 9.5 months in patients with high-PD-L1, (23.6 months vs 14.1 months, HR=0.56 [95% CI: 0.32-0.99]. No improvement in OS was seen in remaining patients with low [12.4 months vs 16.6 months, HR=1.34 [95% CI: 0.95–1.90]] or no PD-L1 expression (13.8 months vs 12.5 months, HR=0.86 [95% CI: 0.65–1.15]) [10].
Non squamous NSCLC
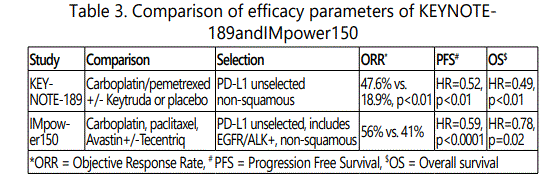
Pembrolizumab [Keynote-189] as well as atezolizumab [IMpower-150] have been evaluated in combination with pemetrexed containing platinum doublet in advanced nonsquamous NSCLC [Table-3]. Both are associated with improved outcome.
Pembrolizumab
In the KEYNOTE–189 study combination of pembrolizumab withchemotherapy (carboplatin + pemetrexed), versus chemotherapy alone was evaluated irrespectiveof PD-L1 expression. Addition of pembrolizumabto chemotherapy resulted in improved ORRby 28.8% [more thandouble (47.6% vs. 18.9%, p<0.01)]. There was an improvement in PFS [HR=0.52, p<0.01] and OS (HR 0.49; 95% CI: 0.38–0.64; p<0.001) at median follow up 10.5 months compared to patients receiving doublet chemotherapy [11].
Atezolizumab
In the IMpower 150 addition of atezolizumab to systemic therapy consisting carboplatin + paclitaxel + bevacizumab irrespective of PD-L1 expression was evaluated. Patients who progressed on targeted therapies for sensitizing EGFR mutation or ALK translocation as well as intolerant to treatment with one or more approved targeted therapies were also included. Addition of atezolizumab was associated with improvement in ORR by 15% [56% vs. 41%], improved PFS of 1.5 months [(8.3 months vs. 6.8 months, respectively. HR 0.62; 95% CI: 0.52–0.74, p<0.0001)] and OS [HR=0.78, p=0.02] [12].
heckpoint Inhibitors as a Maintenance Therapy
Stage III disease
Durvalumab has been evaluated in responding patients with Stage III disease as a maintenance /consolidation therapy.
Durvalumab
In a randomized study, 713 patients who have not progressed post chemo radiation received durvalumab (n=476) or placebo (n=237) as consolidation/maintenancetherapy following chemo radiation [13]. Those who received durvalumab showed improvement in ORR by 12%[26% vs 14%; p<0.001] and median PFS by 11.2 months (16.8 vs. 5.6 months; HR 0.52; 95% CI: 0.42–0.65; p<0.001), Results were consistent across pre-specified demographic and clinical subgroups, including never-smokers, irrespective of baseline PD-L1 tumor expression. The incidence of grade 3/4 adverse events was similar with durvalumab (29.9%) and placebo (26.1%) [13].
Stage IV Disease
Checkpoint inhibitor, responsible for response in a given patient, is recommended for use as a maintenance therapy. E.g. pembrolizumab, if its use was associated with response to therapy.
Checkpoint Inhibitors as a Second Line Therapy
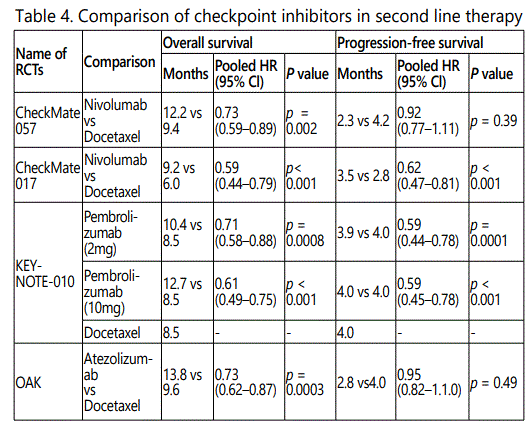
Checkpoint inhibitors (Nivolumab, pembrolizumab, atezolizumab) had their first approval in management of NSCLC as a second line therapy based on improvement in OS [Table-4]. They were evaluated as a monotherapy against docetaxel as chemotherapy. Improvement in OS was not related to changes in PFS [Table-4]. Immunotherapy was found to be less toxic compared to docetaxel in all studies.
Nivolumab
The CheckMate017 evaluated nivolumab in squamous cell NSCLC in 272 patients. Nivolumab improved median OS by 3.2 months (9.2 vs. 6.0 months; HR 0.59; 95% CI: 0.44– 0.79; p<0.001) [14].
The CheckMate 057 evaluated nivolumab in nonsquamous NSCLC in 582 patients. Nivolumab improved median OS by 2.8 months (12.2 months vs. 9.4 months; HR 0.73, 95% CI: 0.60–0.89; p=0.002) [15].
In both studies, nivolumab was found to have a better safety profile (treatment-related AEs grade≥3; ≤10% vs ~55%) [14-15]. Nivolumab 480 mg IV can be given at every 4 weeks [16].
Pembrolizumab
The KEYNOTE-010 evaluated pembrolizumab in 1034 previously treated patients with a PD-L1 TPS ≥1%. It compared pembrolizumab (2 mg/kg or 10 mg/kg) with docetaxel. Pembrolizumab improved median OS in a dose dependent way. (HR 0.71, 95% CI: 0.58–0.88; p=0.001 for 2mg/kg and HR0.61, 95% CI: 0.49–0.75; p<0.001 for 10mg/kg). Pembrolizumab was better tolerated than docetaxel. Grade≥3 adverse events were also less common with pembrolizumab [17].
Atezolizumab
The OAK study evaluated atezolizumab in 850 patients who had progressed following treatment with one or more platinum-containing combination regimens [18]. It improved OS by 4.2 months (13.8 months vs. 9.6 months; HR 0.74, 95% CI: 0.63–0.87; p=0.0004) [18].
Targeted Therapies
Advanced NSCLC with Sensitizing EGFR Mutation First Line Therapy
Erlotinib, gefitinib, afatiniband osimertinib are approved as a first line therapy and are recommended by NCCN guidelines also. Clinical efficacy of erlotinib, gefitinib and afatinib are identical. However, afatinib is more toxic compared to the other two. Osimertinib is found to be more effective and least toxic in a head to head comparative study [19].
Osimertinib
In the Flaura study 556 patients with presence of sensitizing EGFR mutations were randomised to receive osimertinib or gefitinib/erlotinib [22]. The ORR were similar in the two groups (80% with osimertinib and 76% in the SOC group) but response to osimertinib was more durable (17.2 months with Osimertinib vs. 8.5 months with standard EGFR tyrosine kinase inhibitors (TKI) and significantly improved median PFS (18.9 months vs. 10.2 months; HR, 0.46; (95% CI, 0.37 to 0.57; P<0.001). Improvement in PFS was identical with Osimertinib in patients with or without CNS metastases (HR, 0.46vs 0.47). Adverse events of grade 3 or higher were less frequent (34% vs. 45%) with osimertinib compared to gefitinib, erlotinib group [19].
Second Line Therapy
For patients with metastaticEGFRT790M-positiveNSCLC who have progressedon erlotinib, gefitinib,orafatinib. Osimertinib is indicated in preference to chemotherapy. However those who progress on osimertinib, there is no data supporting other TKI and chemotherapy is indicated [5].
Osimertinib was evaluated in the AURA3 study [20]. In AURA study, 419 patients with T790M-positive advanced non-small-cell lung cancer, progressing after first-line EGFRTKI therapy, received osimertinib or chemotherapy (platinum+pemetrexed) in a 2:1 ratio.The median PFS was significantly longer with osimertinib than with chemotherapy. (10.1 months vs. 4.4 months; HR; 0.30; 95% CI], 0.23 to 0.41; P<0.001). The objective response rate was also significantly better with osimertinib (71% vs. 31%; odds ratio for objective response, 5.39; 95% CI, 3.47 to 8.48; P<0.001). In patients with CNS metastases, the median PFS was longer among patients receiving osimertinib compared to those receiving chemotherapy(8.5 months vs. 4.2 months; HR,0.32; 95% CI, 0.21 to 0.49). The proportion of patients with adverse events of grade 3 or higher was lower with osimertinib compared to chemotherapy (23% vs 47%) [20].
Advanced NSCLC with ROS1 Rearrangements
Crizotinib and ceritinib are approved for patients having advanced NSCLC with ROS1 rearrangements. As per NCCN guidelines crizotinib is preferred.
Crizotinib
Crizotinib was evaluated in a single arm study in 50 patients with advanced NSCLC who were positive for ROS1 rearrangements. Objective response rate of 72% (95% CI, 58–84); with 3 complete responses was seen [21]. The median duration of response was17.6 months (95%CI,14.5 tonot reached), and the median PFS was 19.2 months (95%CI, 14.4to not reached). The response was seen irrespective of type of ROS1 rearrangement.
Ceritinib
Ceritinib was evaluated in a single arm study in 28 patients diagnosed to have advanced NSCLC with ROS1rearrangements [22]. One completeresponseand19 partial responses (overall response rate, 62% [95% CI, 45%-77%]) were observed with disease control rate of 81% (95% CI, 65% to 91%). Duration of response was 21.0 months (95% CI, 17 to 25 months). PFS was 19.3 months (95% CI, 1-37 months) for crizotinib-naıve patients and 9.3 months (95% CI, 0-22 months) for all patients. The median overall survival was 24 months (95%CI, 5-43months). Of the eight patients with brain metastases, intracranial disease control was reported in five patients (63%; 95% CI, 31% to 86%). Diarrhoea (78%), nausea (59%), and anorexia (56%) were the most common adverse events, the majority of which mild in nature. [22].
ALK Rearrangements: Metastatic NSCLC who are Positive for ALK Gene Rearrangements
First Line Therapy
Alectinib,crizotinib and ceritinib are approved for first line treatment of advanced NSCLC with ALK gene rearrangement. However, controlled clinical study comparing alectinib and crizotinib revealed alectinib to have better efficacy and safety. Due to this alectinib is preferred over crizotinib.
Alectinib
In the ALEX study, alectinib and crizotinib were evaluated in 303 patients with ALK-positive advanced NSCLC [23] and also included patients with asymptomatic CNS disease. The 12-month event-free survival rate was better for alectinib. (68.4% vs. 48.7%; HR, 0.47 [95% CI, 0.34 to 0.65]; P<0.001)) [23]. The median progression-free survival assessed by an independent review committee was 25.7 months vs. 10.4 months [HR, 0.50 [95% CI, 0.36 to 0.70]; P<0.001)]. Control of CNS metastasis was also better with alectinib than with crizotinib (No. of patient with CNS progression (12% vs 45%) and the time to CNS progression (HR, 0.16; 95% CI, 0.10 to 0.28; P<0.001)). Responserates were identical (82.9% with Alectinib vs 75.5% with crizotinib(P = 0.09)) but duration of response was significantly better with alectinib (HR, 0.36 [95% CI, 0.24 to 0.53).
In the J-ALEX study, 207patientswith ALK-positive advanced NSCL were randomised to receive alectinib or crizotinib. All patients were treatment naïve or had received one previous chemotherapy regimen. Median progression-free survival was significantly better with alectinib compared to crizotinib as a first line therapy [HR 0⋅31 (95% CI 0⋅17-0⋅57)] and second-line treatment HR 0⋅40 (95% CI 0⋅19–0⋅87) [24].
Alectinib was also better tolerated.
Second Line Therapy
Alectinib, ceritinib and brigatinib are found effective as a second line treatment for patients who progress on crizotinib. Of these only ceritinib is evaluated against chemotherapy in a controlled study. Alectinib and brigatinib are evaluated in a single arm study.
Ceritinib
Efficacy and safety of Ceritinib were compared with chemotherapy in 231 patients progressing on crizotinib and chemotherapy in the ASCEND-5 study [25]. Ceritinib showed a significant improvement in median progression-free survival compared with chemotherapy (5⋅4 months vs 1⋅6 months; HR, 0⋅49 [0⋅36-0⋅67]; p<0⋅0001). Treatment-related serious adverse events were similar between groups [11%]. However ceritinib was associated with increased frequency of enzyme elevation.
BRAFV600E Mutations
First Line
Dabrafenib/trametinib combination therapy was evaluated in 36treatment naïve patients with metastatic NSCLC and BRAF V600 Emutations [26]. The overall response rate was 64% (23/36) with 2completeresponses.The median PFS was 10.9 months (95% CI, 7.0–16.6). All patients had at least one adverse event with grade3 or 4 adverse events seen in 25 patients (69%) and included alanine aminotransferase increase(14%[5/36]), pyrexia (11%[4/36]), aspartate aminotransferase increase(8%[3/36]), and ejection fraction decrease(8%[3/36]).
Subsequent Therapy
The combination regimen of dabrafenib/trametinib was evaluated in a single arm study in 57 patients with advanced NSCLC and BRAFV600E mutations who had progressed on chemotherapy [27]. Response was seen in 63% (36/57) of patients. PFS was 9.7 months (6.9-19.6). Serious adverse events occurred in 56% (32/57) of patients. Grade 3 to 4 adverse events included neutropenia in 9% of patients (5/57), hyponatremia in 7% (4/57), and anaemia in 5%(3/57). Preliminary data from an updated analysis of this phase 2 study reported median OS of 18.2 months (95%CI, 14.3- notestimable) in patients receiving dabrafenib/trametinib [28].
Management of Metastatic NSCLC to Reflect Recent Advancements
First-Line Therapy
Patients without Actionable Mutations
Patients with PD-L1 TPS≥50%: Based on current evidence they should be treated with pembrolizumab as a monotherapy [29]. Patients with PD-L1 TPS< 50%: They should receive pembrolizumab + pemetrexed containing therapy or paclitaxel containing therapy based on histology [29].
Patients with Actionable Mutations
Patients with actionable mutations should receive the following preferably depending on the type of actionable mutations. EGFR sensitising mutations: Preferably osimertinib. ROS1 rearrangement: Preferably crizotinib. ALK rearrangement: Preferably alectinib. BRAFV600E mutations: Preferably dabrafenib + trametinib
Second-Line Therapy
Patients without Actionable Mutations
With increasing use of checkpoint inhibitor in first line therapy, they are recommended as a second line only if they were not previously treated with checkpoint inhibitors in patients who are not candidate for targeted therapy. Nivolumab, atezolizumab are preferred over pembrolizumab to avoid evaluation of PD-L1 expression [29].
Patients with actionable mutations
Patients with actionable mutations should receive following as a subsequent therapy depending on first line therapy received. EGFR sensitising mutations: Osimertinib if first line therapy was erlotinib, gefitinib or afatinib. Chemotherapy if firstline therapy was osimertinib. ALK rearrangement: Alectinib, ceritinib or brigatinib if first line therapy was crizotinib. BRAFV600E mutations: Preferably dabrafenib+trametinib. In all other patients chemotherapy is advised as second line therapy.
Discussion
Till recently, advances in the management of advanced NSCLC did not improve outcome significantly and NSCLC continued to be the major cause of cancer related mortality. Recent advances in targeted therapy and introduction of immunotherapy have changed this by providing more options and durable responses.
Introduction of checkpoint inhibitors has changed the scenario dramatically. They provide additional options to treat advanced NSCLC. Their use as a monotherapy as well as in combination with chemotherapy is associated with improved response rate, progression free survival and overall survival. The responses seen with checkpoint inhibitors are more durable than those seen with other therapies in management of NSCLC. Use of pembrolizumab in the first line management is associated with better outcome compared to conventional therapy as well as other checkpoint inhibitors. Pembrolizumab improves objective response rate as well as one year overall survival by around 20% compared to control arm. Use of checkpoint inhibitors brings in novel side effects in the form of auto-immune reaction. Checkpoint inhibitors are better tolerated than chemotherapy when used as a monotherapy. When combined with chemotherapy, checkpoint inhibitors do not increase side effect profile of the chemotherapy.
In spite of their advantages, checkpoint inhibitors are not widely used for treatment of NSCLC around the world due to their cost and availability in different parts of world. Introduction of pembrolizumab as the first line is considered to be cost effective in the USA [30] but not in the UK [31]. Similarly, cost of nivolumab in the second line therapy is considered expensive in Canada [32]. Long term data for check point inhibitors are maturing and will also be available. Recently, nivolumab showed five year survival of 16% when used as a second line therapy [33]. It is expected that use of first line pembrolizumab will improve it further. Long term data of better five year survival and availability in remaining parts of the world willimprove the use of checkpoint inhibitors globally.
In patients with the EGFR sensitising mutation, osimertinib has almost doubled PFS [HR=0.46] in comparison with erlotinib and gefitinib. Osimertinib can also be used in the presence of brain metastasis. However cost of therapy of osimertinib is not found cost effective as first line or second line in USA [34-35]. Similarly alectinib has also doubled PFS [HR=0.5] in comparison to crizotinib and replaced crizotinib as a first line therapy. Alectinib is probably the only newly approved drug in the first line management of NSCLC which is found cost effective [36].
Five year survival has been very low and stagnant for patients with advanced NSCLC till recently. With all the advances, we are entering in to a new era in the management of advanced NSCLC, wherein significant improvement in five year survival looks achievable.
Conclusion
There have been significant advancements in first line therapy. Pembrolizumab is suggested as a first line therapy in place of chemotherapy in patients expressing PD-L1≥50%. In rest of patients without actionable driver mutations, it can be combined with appropriate chemotherapy based on tumor histology. For patients with actionable driver mutations; Osimertinib is suggested in patients with EGFR sensitising mutation, Crizotinib for patients with ROS1 rearrangement, Alectinib for patients with ALK rearrangement and dabrafenib+trametinib for patients with BRAFV600E mutations as a first line therapy.
Conflict of Interest
Authors state no conflict of interest.
References