Research Article
Her-2/neu Status in Syrian Women with Breast Carcinoma: A Syrian Cohort Study of more than 10000 Cases
1Department of Medical oncology, Al Bairouni University Cancer Center, Damascus, Syria
2Department of Pathology, Al Mujtahed Hospital, Damascus, Syria
3Department of Pathology, Al Bairouni University Cancer Center, Damascus, Syria
4Al Assad University Hospital, Damascus, Syria
*Corresponding author: Maher Salamoon, Department of Medical oncology, Al Bairouni university cancer center, Damascus, Syria, Tel: +963 933 771086, E-mail: maher.salamoon@gmail.com
Received: July 28, 2018 Accepted: September 3, 2018 Published: September 7, 2018
Citation: Salamoon M, Assad L, Ahmad F, Kenj M, Bachour M. Her-2/neu Status in Syrian Women with Breast Carcinoma: A Syrian Cohort Study of more than 10000 Cases. Madridge J Cancer Stud Res. 2018; 2(1): 65-69. doi: 10.18689/mjcsr-1000109
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Her-2/neu is a member of epidermal growth factor receptors (EGFR) implicated in the pathogenesis of breast cancer. Her-2/neu is known to be overexpressed in 30% of breast cancers.
Objective: the study is aimed at determining the prevalence of Her-2/neu overexpression in Syrian women diagnosed with breast cancer.
Material and Methods: The study included 10355 Syrian women diagnosed with breast cancer (invasive carcinoma NOS) during the period between 2007 and 2012 at Al Bairouni university hospital in Damascus (SYRIA).Both Her-2 (++ and +++) were checked by FISH, [Hercep test (DAKO Inc)] to determine Her-2/neu gene status.
Results and Discussion: 2013 patients were found to be Her-2 positive (+++) forming 19.4% of the studied group, 2000 (19.3%) were (++) equivocal. On the other hand, 62% of patients were Her-2 negative (0 and 1+) scores. A further investigation of the positive and equivocal groups was performed by FISH showing positive results as follows: 1815/2013 of the (3+ patients) and 1143/2000 of the (2+ patients) respectively. In other word 57% of the (++) group were found to be FISH positive versus 9% negative FISH results in the (+++) group.
Conclusions: The prevalence of Her-2/neu in Syrian women having breast cancer in this study is 28.5%. The study reflects the distribution of real Her-2/neu gene expression among the Syrian women since Her-2 amplification was detected by FISH in both ++ and +++ groups.
Keywords: Her-2/neu, Breast Carcinoma, Cohort Study, EGFR.
Introduction
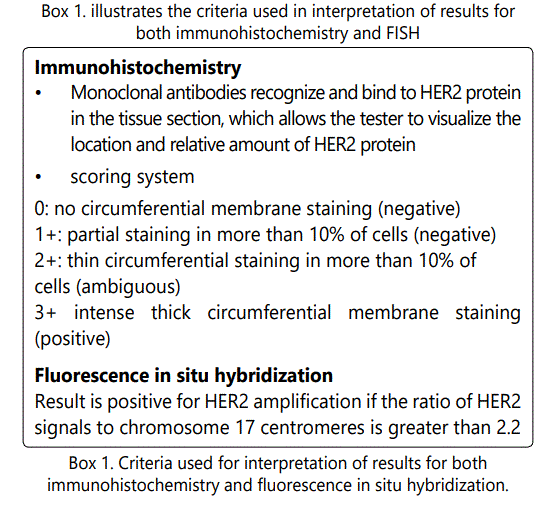
HER-2 is an oncogene that is overexpressed and/or amplified in about one-third of breast cancer cases [1-2]. It is associated with tumor aggressiveness such as high tumor grade, lymph node metastasis, and brain metastasis [3-7]. More importantly, HER-2 is a biomarker for targeted immunotherapy such as with Trastuzumab [8-10]. Therefore, the proper evaluation of HER-2 gene status is important for breast cancer therapy. Trastuzumab therapy improves the survival rate among women with metastatic or localized HER2- positive breast cancer [11-12]. There are 2 tests commonly used to determine HER2 status: immunohistochemistry (detects overexpression of HER2 protein) and fluorescence in situ hybridization (detects amplification of the HER2 gene). A widely recommended testing algorithm is to consider an immunohistochemistry score of 3+ as positive, a score of 0 or 1+ as negative, and a score of 2+ as ambiguous and requiring confirmation with fluorescence in situ hybridization (FISH) [13]. Trastuzumab therapy was approved for use in women with either an immunohistochemistry score of 3+ or a positive fluorescence in situ hybridization result. Studies that showed fluorescence in situ.
Hybridization to be more sensitive than immunohistochemistry in determining HER2 status [14-15] and a retrospective analysis that showed Trastuzumab therapy to be beneficial only in patients who receive a positive result of fluorescence in situ hybridization have raised the issue of whether fluorescence in situ hybridization should be used to determine the HER2 status of all women with breast cancer. In some countries (e.g., Belgium), only patients with a positive result of fluorescence in situ hybridization receive Trastuzumab therapy [16]. Immunohistochemistry is simple and could be performed in most pathology laboratories. FISH, on the other hand, is only performed in selected laboratories with suitable equipment making the test more reliable and widely considered as the test of choice in Her-2/neu gene status [17].
Materials and Methods
Patients
Only patients with invasive carcinoma (NOS) were included in the study. 11013 breast cancer cases with different histologies, among which 10355 cases of primary invasive breast cancer diagnosed between January 2007 and December 2012 at Al Bairouni university hospital in Damascus (SYRIA). 658 patients presented with different histologies including: fibroadenomas, medullary carcinoma, intraductal carcinoma, myeloid sarcomas, Non-Hodgkin Lymphoma and phylloid tumors. Those 658 patients were excluded from the study because of the lack of Her-2 expression. Al Bairouni university hospital is the central cancer center in Syria where almost all cancer registries are recorded. We retrospectively reviewed reports and image files from FISH studies for the HER- 2 genes. Clinicopathologic parameters such as age at initial diagnosis, histologic type, and histologic grade, stage of disease, estrogen receptor status, progesterone receptor status, and HER-2 IHC results were obtained from pathology reports. The histological grade was assessed using the Nottingham grading system [18]. HER-2 staining was analyzed according to the American Society of Clinical Oncology (ASCO)/ College of American Pathologists (CAP) guidelines as mentioned in Box (1).
FISH Analysis
Before FISH analysis, invasive tumors were examined on hematoxylin-eosin stained slides. FISH was subsequently performed on the tested tumor. FISH was performed using a PathVysion HER-2 DNA Probe Kit (Vysis, Downers Grove, IL, USA) according to the manufacturer's instructions. HER-2 gene copy number on the slides was evaluated using an epifluorescence microscope (Olympus, Tokyo, Japan). At least 100 tumor cell nuclei in three separate regions were investigated for HER-2 and chromosome 17 signals. An absolute HER-2 gene copy number lower than 4 or a HER-2 gene/chromosome 17 copy number ratio (HER-2/Chr17 , or amplification index [AI]) less than 1.8 was considered HER-2 negative. An absolute HER-2 copy number between 4 and 6 or a HER-2/Chr17 ratio between 1.8 and 2.2 was considered HER-2 equivocal. An absolute HER-2 copy number greater than 6 or a HER-2/Chr17 ratio higher than 2.2 was considered HER-2 positive [19]. Chromosome 17 polysomy was defined as a centromeric chromosome 17 spot count of 3.0 or more in at least 80% of tumor cells [20]. High-grade amplification was defined as a HER-2/Chr17 ratio higher than 5.0, and lowgrade amplification was defined as a HER-2/Chr17 ratio greater than 2.2 but less than 5.0 [21, 22]. HER-2 gene heterogeneity (GH) was defined as more than 5% but less than 50% of the analyzed invasive tumor cells having a HER-2/Chr17 ratio higher than 2.2, according to CAP guidelines [23].
Statistical Analysis
Data were processed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
With a mean age of 42 years old at diagnosis, 2013 patients were found to be Her-2 positive (+++) forming 19.4% of the studied group, 2000 (19.3%) were (++) equivocal. On the other hand, 62% of patients were Her-2 negative (0 and 1+) scores. A further investigation of the positive and equivocal groups was performed by FISH showing positive results as follows: 1815/2013 of the (3+ patients) and 1143/2000 of the (2+ patients) respectively. In other word 57% of the (++) group were found to be FISH positive versus 9% negative FISH results in the (+++) group. Detailed information about patient characteristics is illustrated in table (1).
Of the 1958 cases with positive FISH results, Her-2 gene heterogeneity (GH) was observed in 233 (11.8%) cases, all of which were Invasive Ductal Carcinoma (IDC) with higher proportion of borderline Her-2 gene amplification status than other IDC without heterogeneity (P<0.002). The incidence of chromosome 17 polysomy was higher in patients with Her-2 GH group than cases without (P<0.0001). Her-2 amplification associated with intermediate grade (Grade II) in about 50% of cases and accompanied with high tendency of node metastasis.
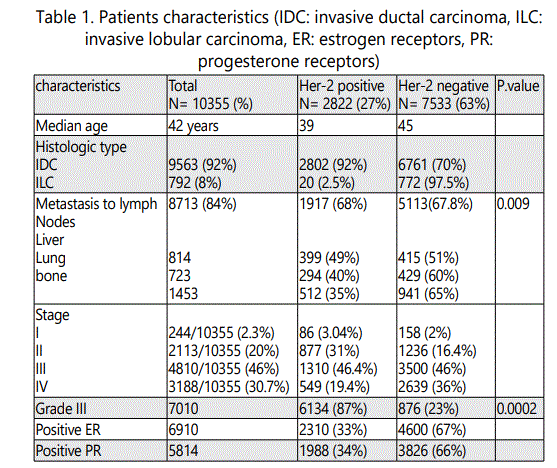
Only 244 patients (2.3%) presented with stage I disease, 2113 patients (20%) presented with stage II disease, 4810 (46%) presented with stage III while the remaining 3188 (30.7%) presented with stage IV disease.
Discussion
Over expression of HER2/neu has been shown to be associated with bad prognosis in patients with node-positive breast cancer and possibly with a node negative breast cancer.
Determination of HER2/neu status appears to be of prognostic and predictive value for patient and physician, and can be readily performed in most hospitals as part of routine clinical assessment for breast cancer patients. There is evidence that women in whom tumor over express HER2/neu are likely to get greater benefit from therapy with anthracycline-containing regimens than from alkylating agents. It is therefore, reasonable to assess HER2/neu on all primary breast tumors at the time of diagnosis [28].
The availability of an efficacious, yet expensive, treatment for HER2-positive breast cancer underlines the need for accurate determination of HER2 status. Our study concluded more than 10000 patients with breast invasive carcinoma showing Her-2/neu gene amplification in 2958/10355 (28.5%) of cases which reflects the accurate percentage because even 3+ cases were evaluated as well as cases with equivocal results by FISH. In the course of study, we noticed that the (+++) status of Her-2 expression by immunohistochemistry was seen to be negative in 9 % of cases. The former result is very important from both clinical and economic point of view, because Trastuzumab is given to all patient in that group (+++ status) according to treatment guidelines and irrespective of Her-2 gene status by FISH, the thing that enable us to give the medication in the proper indication and save money as well.
Breast cancer GH results from clonal diversification and differences in genetic composition from growing genetic instability in some part of the tumor [24, 25]. In our study, Her-2/neu GH was observed in 8.25% of all studied cases with positive Her-2. Genetic differences in a single tumor were shown by laser microdissection and a comparative genomic hybridization study, and these differences were confirmed by FISH and microsatellite instability analysis [26]. HER-2 GH could be explained by acquisition or loss of the HER-2 gene, but HER-2 gene amplification is more plausible, considering that HER-2 is associated with tumor aggressiveness [27]. Therefore, Her-2/neu GH needs careful study and interpretation of FISH because signals are not homogenous on all areas of the slide.
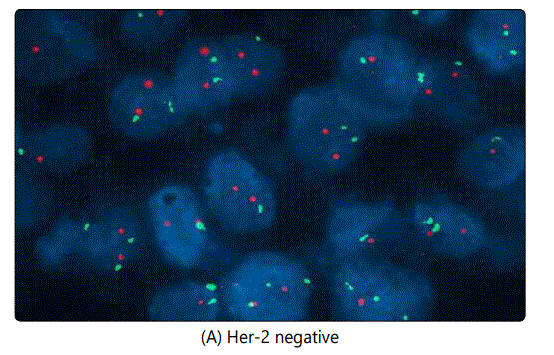
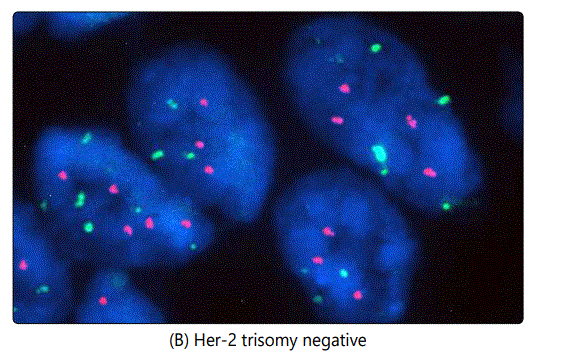
Another issue encountered in the study is the case of chromosome 17 polysomy which was observed in 86 out of 2958 (2.9%) of Her-2 positive cases, an example is illustrated in image (G). In this case, we do not know exactly when and where to give Trastuzumab, however Trastuzumab was not offered for those patients according to our own guidelines. In some cases we noticed both Her-2 positive and negative trisomy, also Her-2 positive pentasomy and hexasomy were seen in some patients as well (as seen in images H and F respectively). Her-2 positive and negative trisomy was also observed (5 cases positive and 21 negative as illustrated in images (E) and (B) respectively.
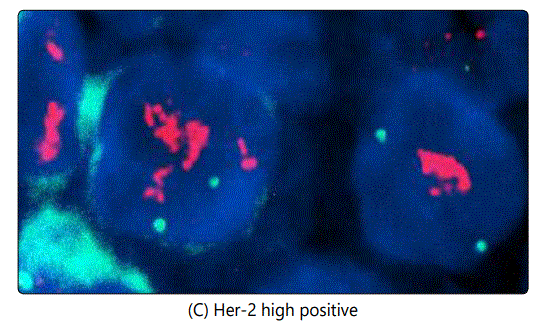
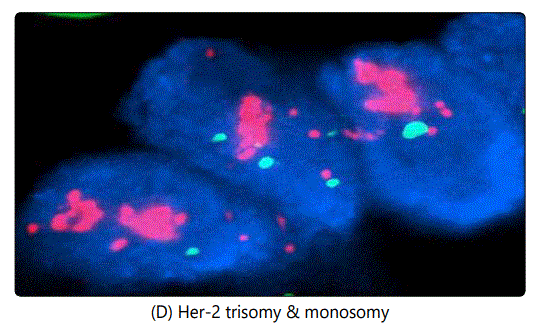
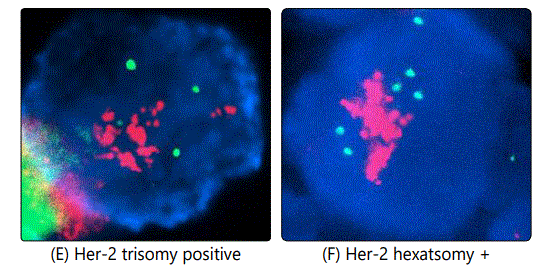
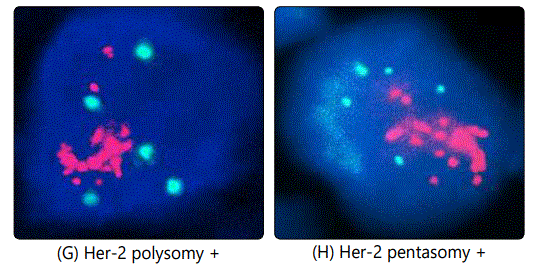
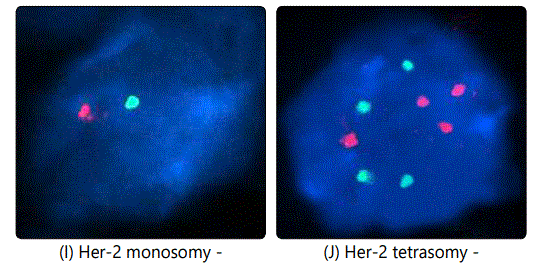
In this study, we reported about 300 cases with Her-2 GH and to avoid interpretation bias, we followed ASCO guidelines by scanning the entire slide (scanning more than 4 fields) [27]. Finally, the incidence of Her-2 in Syria seems to be similar to the international prevalence (20-30%), however, documented positive cases were confirmed by FISH, making our results more trustable. therefore, Trastuzumab is not indicated in Syria unless Her-2 is positive in both ++ and +++ status.
Acknowledgement
We thank Al Bairouni cancer center for support and Kenj cytogenetic lab for technical support and review of some cases.
Conflict of Interest
Authors state no conflict of interest.
References