Research Article
Somatic Embryogenesis in Hyacinth in-vitro Culture
1 Associate Professor, Development geology, Department of Biotechnology, Batumi Shota Rustaveli State University, Georgia
2 Professor, Department of Genetics, Batumi Shota Rustaveli State University, Georgia
3 Science-worker, Department of Biotechnology, Batumi Shota Rustaveli State University, Georgia
4Assistant, Department of Biotechnology, Batumi Shota Rustaveli State University, Georgia
5Associate Professor, Conservation, Plant ecology, Department of Botany, Batumi Shota Rustaveli State University, Georgia
*Corresponding author: Nana Zarnadze, Associate Professor, Department of Biotechnology, Batumi Shota Rustaveli State University, Georgia, E-mail: z_nana@mail.ru
Received: November 13, 2018 Accepted: December 20, 2018 Published: January 7, 2019
Citation: Zarnadze N, Dolidze K, Manjgaladze S, Bolkvadze T, Diasamidze I. Somatic Embryogenesis in Hyacinth in-vitro Culture. Int J Biotechnol Recent Adv. 2019; 1(2): 57-59. doi: 10.18689/ijbr-1000109
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This work covers peculiarities of introduction of the hybrid form of hyacinth (Hyacinthus) introduced and spread in Georgia into in-vitro culture and induction of embryogenesis. Aiming to derive the sterile cultures, we matched the sterilizing agent and its exposition. Besides, we used hyacinth bulb peel as an initial explant to derive the embryogenic callus. Aiming induction of callusogenesis and embryogenesis, we tested and researched concurrent exposition to benzylaminopurine and auxins (NAA and IBA) in concentrations correlation of 2:10; 2:15; 2:20. We derived the embryogenic calli and somatic embryoids, developed conditions for maturation of the embryoids and regeneration of the somatic embryos.
Keywords: Somatic embryogenesis; Cell culture; Totipotency; Auxin; Cytokinin; Callus
Introduction
Totipotency is one of the important properties of the plant somatic cell in the in-vitro culture. It means the cells capacity of implementation of genetic program, i.e. spring and originate an entire plant [1,2]. Plant cells in tissue culture exercise this property by the various ways, including induction of somatic embryogenesis, i.e. origination of embryo like formations - embryoids, somatic embryos from the isolated or several somatic cell groups. Somatic embryos develop asexually, without embryo sac, in three steps according to Steward: globular, heart-shaped and torpedo-like ones. Finally they have the tend of gemma development [3,4]. Embryoid is a bipolar structure with buds and root developing under effect of own or supplied hormone-regulating system and going the stages generally typical for zygote embryo. Embryoids develop in tissue culture resulted dedifferentiation of explants cells, on the callus or directly on the first explants [1]. Induction of each of them is regulated with the phytohormones and concentration of nutrient medium components [2,5].
Most of the regenerate plants derived in the tissue culture by the way of somatic embryogenesis, mainly differ from the parent plant according to one or several marks, due to which they are selectable [4,6].
Currently, this method is effectively used for reproduction of many bulbous and various woody plants. In spite of this, the main difficulty in induction of plants by the way of somatic embryogenesis in cell culture is that it is enough difficult to switch a somatic cell over to a somatic embryoid and derive and maturate the full-value somatic embryos [1]. Studies on factors, controlling in vitro plant morphogenesis, are highly desirable not only for the development of improved regeneration systems, but also for the analysis of molecular mechanisms underlying plant embryogenesis [7,8].
The aim of our work included introduction of the hybrid form of hyacinth (Hyacinthus) introduced and spread in Georgia into in-vitro culture and research of peculiarities of morphogenesis thereof.
For realization of the results the following problems were to be solved:
Object and Method of Research
Hyacinth is a perennial bulbous plant of Convallariaceae family. Various cultivars of hyacinths have the flowers of various colour, size and shape. They are cultivated on well-lighted, fertile loamy soil. It is cultivated by bulbs, rarely by seeds. Oil produced from hyacinth flowers is used in perfumery. Hyacinths are the best hothouse and bench plant.
For production of sterile explants we used the plants cultivated in hothouse. The initial material was presented by the bulbs. For the further steps of micro reproduction we used invitro cultivated plant organs. We peeled the hyacinth bulbs and placed them in the diocidum 0.2% water solution for superficial sterilization, providing exposition of 10-20 minutes. During further 8-10 days we placed the sterile bulbs on the agar nutrient medium, divided the sterile bulbs into fragments and planted them on the hormone-containing nutrient mediums.
Nutrient medium - Gamborg B5 [9] medium containing mineral salts. According to the experiment, we added to the medium the growth regulators for development of plants. Cultivation was provided in the phytotron. Lighting 2-3 Lux, T 27 ± 1°C, photoperiod 16/8 hours. For induction of calluso genesis, the explants were placed in dark thermostat at 25 ± 1°C temperature. Incubation of calli placed on the organogenic nutrient medium was provided in phototron at the above conditions.
We started registration of the sterile material from the third day and expressed the results in percents. Embryogenic potential of the cultivated material was assessed after 40-50 days from plating of the cultures by the way of determination of quantity of embryo structures derived at each explant. Statistic processing was provided in Excel program.
The Results of the Experiment and Discussion
The experiment results proved that sterilizing agents exposition was differ in respect of aseptic cultures output and viable cultures production. The maximum result was received by the way of sterilization by 0.2% water solution of diocidum providing 10 munities exposition. Sterilization degree was 90%, viability - 95%.
For production of the embryogenic callus, we used hyacinth bulb peels as the initial explants to receive the embryogenic callus. Aiming induction of callusogenesis and embryogenesis, we tested and researched concurrent exposition to benzylaminopurine and auxins (NAA and IBA) in concentrations correlation of 2:10; 2:15; 2:20.
The experiments results showed that the both researched auxins caused induction of callus from the named explants. Depending of the kind of growth regulator introduced in the zero passage nutrient medium, the calli of different morphology and etiology formed. Addition of the auxin (namely IBA) together with BAP into the nutrient medium caused development of the middle growth rate callus. The calli had dense, angular structure. Morphogenetic potential of these calli included rhizogenesis only.
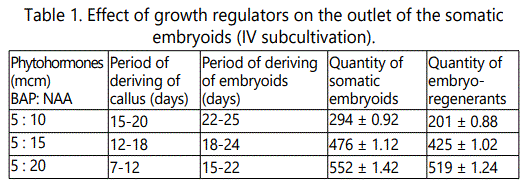
Absolutely different response of the explants occurred in case of concurrent introduction of naphthylacetic acid and benzylaminopurine. Callus induction occurred the 7th-10th day after plating of the explant, starting from the peripheral parts to the center. The 12th-15th day, it completely covered the explants and on 18th-20th day - the whole cultivation dish. The callus had friable consistence, all the introduced explants intensively callused at the concentrations of the hormones supplied to the nutrient medium (Figure 1). We divided the well-growing callus mass into 2-3 parts and transferred to the nutrient medium of the same hormone content. Further subcultivation was provided at faint lighting. During the 3rd cultivation so called aggregates began to form on the calluses. During the 4th subcultivation, the aggregates stretched originating the embryogenic pipes, at the ends of which the isolated embryosomatic initials originated. They intensively divided and the process finished with formation of the globular somatic embryos (Figure 2). Frequency of origination of the somatic embryos depended on content of the nutrient medium including concentration of the matched hormones and content of the mineral salts (Table 1).
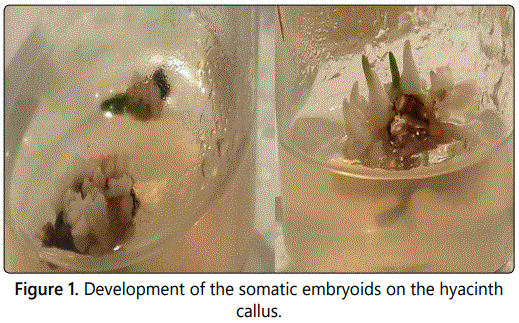

The following stage is presented with maturation of the somatic embryoids. It is enough complicated process, as the embryogenesis is asynchronic. Origination and appropriately maturation of the embryoids are not simultaneous. Sometimes, in the process of sub cultivation we observed destruction of the small part of the embryogenic cells due to disintegration to cells. Besides, concentrations of phytohormones in the inducing and maturating mediums mismatched. We maturated the somatic embryioids on the nutrient medium containing the even concentration of benzylaminopurine and naphthylacetic acid (5:5 mcm). During 15-18 days the observed embryoids growing in volume, simultaneous development of lobular tissue in conical direction. After 30 days we derived the somatic embryos of bipolar structure (Figure 1). We transferred them to the organogenic nutrient medium containing only one phytohormone, benzylaminopurine in low concentration (5 mcm). During 10 days the buds developed from the apical part of the embryo (Figure 2), but development of the rootlet was limited under the effect of cytokinin. As for the embryos transferred to the nutrient medium not containing any hormones, both buds and rootlets developed.
The process of development of gemma from the somatic embryoids was similar to the process of origination of sprout from the seed, what makes us consider that the embryoids originated from the somatic embryoids had the zygotic embyo-like structure. For microreproduction of the regenerated buds we transferred them to the nutrient medium containing benzylaminopurine of 8-10 mcm concentration, but a part of them we rotted on mineral content nutrient medium being ½ of the Gamborg medium under effect of 4 mcm indole-3-butyric acid. In the process of rotting we observed development of mictrobulbs on the rootlets.
Conclusions
So: we produced aseptic cultures of hyacinth; Developed the hormone content of the nutrient medium for induction of embryogenic callus cultures; Callus tissues were highly embryogenic and preserved this feature for two and more year's period; Embryoids originated and maturated on the callus tissue acynchronically; We developed the nutrient mediums adequate for maturating and regeneration of the embryoids. The produced embryo cultures well reproduced and rooted providing origination of mictrobuds on the rootlets.
References