Research Article
Histological and Biochemical Features of the Digestive System in the Cage-Reared Gilthead Sea Bream (Sparus aurata)
1Department of Biology, Faculty of Science, University of Split, Ruđera Boškovića 33, Split, Croatia
2Faculty of Humanities and Social Sciences, University of Split, Poljičkacesta 35, Split, Croatia
*Corresponding author: Ivana Bočina, Department of Biology, Faculty of Science, University of Split, Ruđera Boškovića 33, 2100, Split, Croatia, E-mail: bocina@pmfst.hr
Received: November 22, 2018 Accepted: December 12, 2018 Published: December 21, 2018
Citation: Pavelin T, Kević N, Restović I, Bočina I. Histological and Biochemical Features of the Digestive System in the Cage-Reared Gilthead Sea Bream (Sparus aurata). Int J Biotechnol Recent Adv. 2018; 1(2): 51-56. doi: 10.18689/ijbr-1000108
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Five adult cage-reared gilthead sea bream Sparus aurata specimens were sampled to study the histological and biochemical features of its digestive system. Staining using Hematoxylin-eosin, Alcian blue and Orcein were used to elucidate the histological and biochemical features of the digestive organs in cage-reared gilthead sea bream. The digestive system is a hollow tube made of esophagus, stomach and intestines. The esophagus consists of four layers: mucosa, sub-mucosa, muscular layer and the outer layer. The esophageal epithelium contains abundant goblet cells with alkaline or neutral mucopolysaccharides (MPS) inside them. The wall of the fundic and pyloric region of the stomach is also four-layered and goblet cells of the stomach mucosa contain acid MPS. Four pyloric caeca were found between stomach and the intestines. According to its morphology the intestines of gilthead sea bream could be distinguished in three parts: anterior, middle and posterior intestines. In spite the difference in their diameter all three parts of the intestines has shown certain similarity regarding histological features. Goblet cells containing acid MPS were found in all three parts of the intestines although they are more numerous in the upper intestines. The liver in gilthead sea bream consists of two lobes. The structure of the hepatocytes is damaged due to large amounts of fat inside the cells. Pancreatic tissue is scattered down the length of the intestines as well as throughout the liver parenchyma. It contains zymogene cells arranged in serous acing. It can be concluded that the histological and biochemical of the digestive system in cage-reared gilthead sea bream is mostly like in other carnivorous fishes and congruent to its feeding habits.
Keywords: Gilthead Sea Bream; Digestive system; Histology; Biochemistry.
Introduction
The gilthead seabream Sparus aurata (Linnaeus, 1758) is a member of subtropical Sparidae family and it could be found in the Mediterranean and the Black Sea, as well as in the Eastern Atlantic, from the British Isles, Strait of Gibraltar to Cape Verde and around the Canary Islands [1]. In the Adriatic Sea the gilthead seabream is wide spread all over the coast, mostly near estuarine [2], inhabiting sandy and rocky-sandy bottoms mostly between 5 to10 m in depth [3]. Commercial breeding of gilthead seabream began in the 1980s, spreading from Italy and France throughout the other Mediterranean countries. At this moment, seabream and the European sea bass Dicentrarchus labrax are considered the most significant fish aquaculture goods in the Mediterranean [4]. In contrast to the other fish aquaculture in Croatia, the gilthead seabream is relatively less produced compared to the other Mediterranean countries. The gilthead seabream feeds mainly on molluscs and crustaceans [5]. For the last ten years, a significant growth in wild gilthead seabream population has been documented [6] that has an opposing consequence on shellfish culture by preying on Mediterranean mussels, Mytilus galloprovincialis, and the European flat oyster, Ostrea edulis [7]. The present study provides first information on the histological and biochemical features of the digestive system in cage-reared gilthead sea bream. The alimentary tract in fish, as in other vertebrates, is made up of alimentary canal, a hollow tube of fluctuating diameter which is longitudinally divided into the esophagus, the stomach, the intestines, and the rectum [8]. The tongue and the teeth in the oral cavity are also included as associated organs in the digestive system, and the extramural digestive organs: liver, gallbladder and pancreas. Salivary glands, in most cases, are missing in the oral cavity of fish [8]. From the cranial end of the esophagus to the caudal end of the rectum, histologically, the wall of the digestive tube consists of four distinctive layers. When starting from the lumen, these layers are: the mucosa, the submucosa, the muscularis externa, and the serosa or adventitia [9]. Although, the histological features of the digestive tract in the wild gilthead seabream was previously studied [10] as well as its morphology of the tongue [11]. The aspects of the histological and biochemical of the cage-reared gilthead seabream were lacking. Consequently, the aim of this study was to reveal the histological and biochemical of digestive system in cagereared (Sparus aurata).
Materials and Methods
A total of ten adult gilthead seabream Sparus aurata specimens were sampled to study the histological and biochemical features of digestive system. The specimens were collected on the fish farm in Peleš Bay near Zečevo, Croatia in April 2018. Immediately after collecting, parts of the digestive tube were fixed in 10% formalin. After few day fixations, the tissues samples underwent dehydration in an ascending series of ethanol, starting from 50% ethanol. Tissue samples were then cleared with xylene and embedded in paraffin. Tissue sections were cut transversally at 6 μm and mounted on glass slides. After deparaffinization in xylene, tissue sections were stained with haematoxylin-eosin staining to present basic morphology of the digestive tube, and then with Alcian blue/PAS and Orcein stain to elucidate its histochemical composition [12]. The slides were observed using a ZeissAxioskop light microscope.
Alcian blue/PAS staining
This staining was used to differentiate MPS in the digestive system of Sparus aurata [12,13]. When using Alcian blue/PAS staining a certain number of glass slides were also stained with haematoxylin. The slides were first deparaffinised and stained with Alcian blue dye for 30 minutes. Subsequently, slides were washed in distilled water and treated with periodic acid for ten minutes and then with Schiff's reagent for ten minutes. The sections were then dehydrated, cleared and mounted on glass slides [13].
Orcein staining
Orcein staining was used to observe the presence of elastic fibers in tissue samples. After deparaffinization, tissue sections were stained with Orcein dye for an hour. After rinsing the sections were left in Giemsa solution overnight. The tissue sections were then stained with eosin for two minutes and then dehydrated, cleared and mounted on glass slides.
Results
The tongue
The tongue's mucosa is lined by a stratified squamous epithelium. The epithelium lies directly on the thin connective tissue rich in blood vessels (figure 1a). Underneath the thin connective tissue an abundant fat tissue as well as cartilaginous tissue could be found. The cartilaginous tissue consists of dense packed chondrocytes surrounded by scarce extracellular matrix. When treated with Orcein dye, the cartilaginous extracellular matrix stained brown to black indicating the presence of elastic fibers (Figure 1b).
The esophagus
The esophageal wall of the gilthead seabream S. aurata consists of four layers: mucosa (tunica mucosa), submucosa (tunica submucosa), muscular layer (tunica muscularis) and the outer layer (Figure 1c).
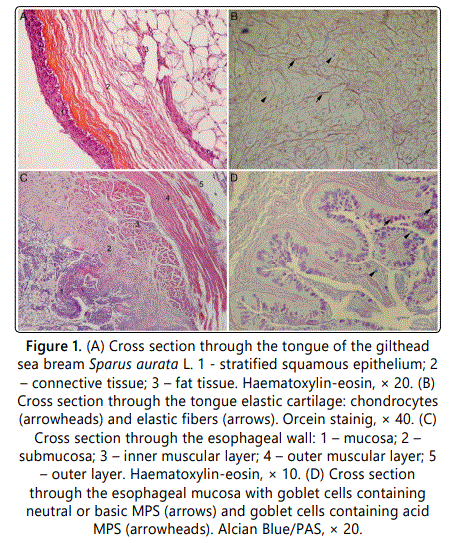
The mucosa is lined with a simple columnar epithelium. The nuclei of the columnar cells are mostly rounded and located at the basal domain of the cell. Large and bubble-like goblet cells are inserted in between epithelial cells. Although most of the goblet cells were stained red with Alcian Blue/PAS staining indicating the presence of the alkaline or neutral MPS, some blue stained goblet cells were also seen in the esophageal mucosa (figure 1d). Lamina propria lies just beneath the epithelium, both deeply is protruding into the esophageal lumen. It consists of a thick connective tissue layer which contains blood vessels. Submucosal layers are very similar in structure to lamina propria. The muscular layer of the esophagus consists of two layers: an inner longitudinal layer and an outer circular layer. The outer muscular layer is made of cross-striated muscle fibers. The outermost layer is formed of thin connective tissue containing blood vessels.
The stomach
The stomach of the gilthead seabream is a sac-like organ placed between the esophagus and intestines. It is possible to distinguish four basic layers in the stomach fundic wall: mucosa, submucosa, muscular layer and the outer layer. The mucosa forms profound folds and contains gastric glands. Mucosa is lined with simple columnar epithelium which coats the opening of the gastric glands (Figure 2a).
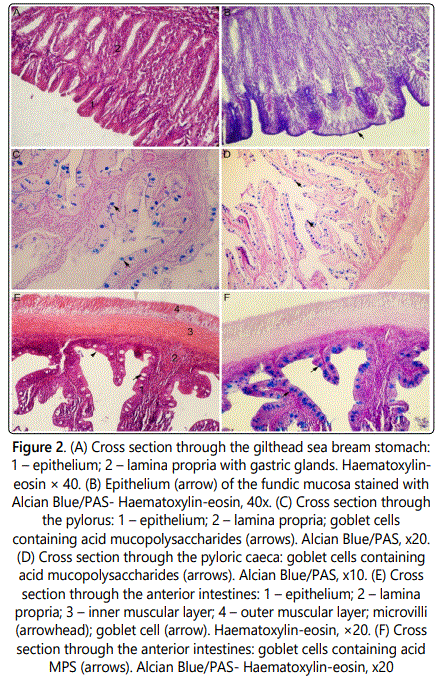
The gastric glands are mostly distributed through the lamina propria with predominance of two cell types; cells with eccentrically located, round-shaped nuclei and cells with elongated nuclei and granulated cytoplasm. The epithelial cells stained purple when using Alcian blue/PAS staining (Figure 2b). Submucosa is a thick layer of connective tissue containing blood vessels. It could be seen on the cross sections that blood vessels are coated by lipid layer at their luminal surface, probably due to feeding habits of the cagereared fishes. The muscular layer of the stomach comprises of two wide muscular layers of smooth muscle cells. The inner muscular layer is circular while the outer muscular layer is longitudinal in structure. A thin mesothelial layer covers the surface of the stomach. In the pyloric region of the stomach, mucosa forms uneven simple leaf-like and branched folds. The mucosal folds are lined by a simple columnar epithelium taller than those of stomach fundus. The columnar cells nuclei are located at the basal domain of the cell. Numerous goblet cells are inserted amongst epithelium cells. When treated with Alcian blue/PAS staining, goblet cells predominantly stained blue indicating the presence of the acid MPS (figure 2c). Submucosa is a layer of dense connective tissue with numerous blood vessels. Muscular layer of the pyloric region contains two layers made of smooth muscular cells: the inner circular layer and the outer longitudinal layer. The outermost layer (tunica serosa) is lined with the simple mesothelial layer. Four pyloric caeca could be found between stomach and the anterior intestines. When using Alcian Blue/PAS staining mucosal goblet cells of pyloric caeca also stained blue due to acid MPS inside them (Figure 2d).
The intestines
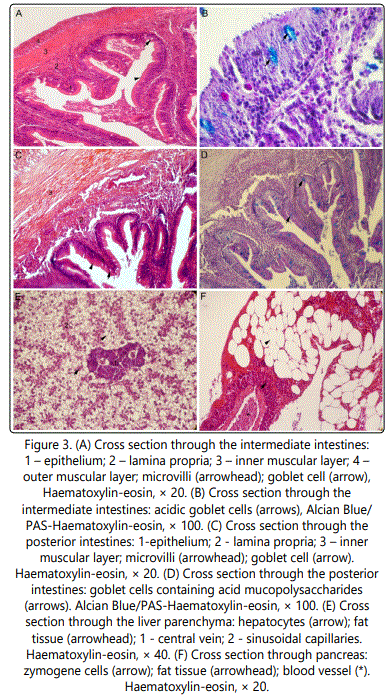
According to its morphology the intestines of gilthead seabream could be distinguished in three parts: anterior, middle and posterior intestines (Figure 3). In spite the difference in their diameter all three parts of the intestines as shown certain similarity in their histological features. The wall of the intestines could be distinguished into four layers: mucosa, submucosa, muscular layer and the outer layer (Figures 3a-3c). The intestines wall is much thinner in comparison with the esophagus and the stomach wall. Mucosa and submucosa of the anterior intestines form high folds deeply protruding into the lumen. The mucosa is lined by a simple columnar epithelium with microvilli. Goblet cells are inserted amongst the epithelial columnar cells. When stained with Alcian blue/PAS goblet cells stained blue indicating the presence of the acid MPS (figure 3f). Goblet cells containing acid MPS could be also found in the middle and posterior intestines although they are not as numerous as in upper intestines (Figure 3b, 3d).
Because of their similarity in structure it is very hard to differentiate sub-mucosal layer from the lamina propria in the sections through the intestines. The muscular layer of the anterior intestinal wall consists of two layers: inner circular and outer oblique muscular layer. One-layered mesothelium covers the outer wall of the intestine. Mucosa of the middle and posterior intestines is also lined with a simple columnar epithelium with microvilli. Submucosa and lamina propria are of the same histological structure. The muscle layer of anterior and middle intestines is made of two layers: the inner circular and the outer longitudinal layer. In the posterior intestines the muscle layer is much wider than in anterior and middle ones. The outermost layer of middle and posterior intestines is similar with those of anterior intestines.
The liver
The liver in gilthead seabream consists of two lobes. The liver cells, hepatocytes, form irregular plates separated from each other by sinusoids and arranged radially around a central vein. The structure of the hepatocytes is damaged due to large amounts of fat inside the cells. Throughout the liver parenchyma scattered pancreas tissue could be distinguished (Figure 3e).
The pancreas
Pancreatic tissue is scattered down the length of the intestines as well as throughout the liver parenchyma. It contains zymogene cells arranged in serous acini. The cytoplasm of these cells stained purple in its basal domain while red granules could be distinguished in the apical domain (figure 3f). This structure is typical for exocrine pancreatic cells found in vertebrates. When treated with Alcian blue/PAS staining, pancreatic cells do not show any typical labelling. Numerous blood vessels as well as adipocytes could be found throughout the pancreatic tissue.
Discussion
The gilthead seabream is economically a very significant aquaculture fish species in Greece and in the general Mediterranean area [14]. Intensive production of Sparus aurata caused market saturation, price crisis and has raised concerns over the quality of cultured gilthead seabream, particularly in comparison with wild gilthead seabream species [14]. The digestive system in vertebrates consists of the alimentary canal and associated organs such as liver, gallbladder and pancreas [15]. The morphology of the digestive tract in fishes is usually in accordance with differences in their feeding habits, as was studied in numerous fish species [10,16-21]. The structure of the intestines is usually consistent among vertebrates, but those of stomach shows remarkable diversity [22]. Some of freshwater and marine fishes such as the Cyprinidae, Labridae and Gobiidae, have even evolutionarily lost their stomach [23]. It is difficult to understand how stomach less fishes digest food, especially the carnivorous ones with great protein uptake in their diet [24], but it seems that this loss does not influence the digestion, as the stomach less fish can be either herbivorous or carnivorous. The herbivorous fishes usually have relatively long guts, whereas in carnivorous fishes the guts are generally shorter than their body length [25-27]. Generally, the jaw size, intestinal length, reproduction and distribution are in relationship to trophic changes and to intraspecific resource partitioning [28].
The oral cavity in fishes usually contains teeth and tongue. The tongue is fixed and composed of a hyaline substance with a pseudostratified epithelium, mostly composed of goblet cells with a few taste-buds [16]. According to Abbate et al. [11], the tongue of the wild gilthead seabream is formed of an enveloping mucosa, a musculature and an osteo-fibrous skeleton. As was shown in the present study, the mucosa of the tongue in cage-reared gilthead seabream is lined with stratified squamous epithelium with underlying connective tissue containing fat and cartilaginous tissue. Since the gilthead seabream is a carnivorous fish, its esophagus is short, muscular tube conducting the food to the stomach. The esophagus of various teleost fish, like those of most vertebrates, functions in transporting food particles, so it is provided with cells that secrete mucus and stratified squamous epithelium [29]. The esophagus of the wild seabream S. aurata shows a multilayered mucosa in the upper part and single-layered regions in the lower part. The single-layered regions are formed by simple columnar epithelium with short microvilli, with no taste buds or mucus-secreting cells and no acidophilic granulated cells [10]. The esophagus of the caged gilthead seabream, as shown in the present study, is layered with single columnar epithelium with inserted goblet cells mostly containing basic or neutral mucins. The mucosa of esophagus in gilthead seabream also contains lamina propria and lamina muscularis mucosae, even though the last one is typically absent in fish [30]. In the European hake Merluccius merluccius, the esophagus is covered by stratified squamous epithelium [20], while in the fresh water stingray Himantura signifier the esophagus is lined with stratified columnar epithelium [31]. In the common eel Anguilla the esophagus mucosa consisted of stratified epithelium, columnar epithelium and goblet cells, while striated muscle fibers formed the thick muscular coat [17].
In vertebrates the stomach is a sac-like muscular organ, separated from esophagus and intestines by sphincters [15]. The present study shows that the stomach mucosa of gilthead sea bream is lined with simple columnar epithelium and it contains gastric glands. The stomach of the wild Sparus aurata is also lined with simple columnar epithelium [10]. In the stomach of Solea senegalensis gastric glands are abundant in the fundic and pyloric regions but absent in the cardiac region [32]. The pyloric region of the cage-reared gilthead seabream is four-layered and it is lined with simple columnar epithelium. As usual in vertebrates, pyloric region is characterized by its pyloric sphincter, a strong circular muscle enabling food particles to pass towards the intestine [18,31,33]. According to present data, fish intestines typically consist of proximal and distal portions [29,31,34]. Since they differ in their morphology, the intestines of the gilthead seabream could be distinguished into three parts: the anterior, middle and posterior intestines. The diameter of the intestines is reducing from the anterior intestine to the posterior intestine. The intestines mucosa is lined with simple columnar epithelium with goblet cells and microvilli. It seems that the intestinal goblet cells in all parts of intestines contain acidic mucins. This is in accordance with Cataldi et al. [10] and indicates the same contents of MPS inside the goblet cells of upper and lower parts of the intestines. Goblet cells with acidic contents in the intestines were also found in some other fish such as European hake Merluccius merluccius [20] and sea bream Mylio cuvieri [29]. It seems that the quality and quantity of the mucus from the intestinal goblet cells is correlated to environmental pollution [35]. The liver in fish is typically a unilobular organ [16,17] formed by a web of epithelial cells, through which a network of vessels and sinusoids runs [10]. The present study has shown that the liver of cage-reared S. aurata consists of two lobes. The liver of garfish Belone contains one simple lobe [21], while the liver of Merluccius merluccius consists of three lobes [20]. The hepatocytes of the liver parenchyma in cage-reared gilthead sea bream are damaged due to high accumulation of fat inside them. Accumulation of fat inside the hepatocytes is probably caused by high fat uptake in the diet of the cage-reared fish. Pancreatic tissue is spread throughout the liver parenchyma and intestines. When compared to other fishes, the digestive system of the cage-reared gilthead sea bream Sparus aurata is mostly in accordance to those of other carnivorous fish as well as with its feeding habits.
Conclusions
The present study has shown that the histological and biochemical features of the digestive system in cage-reared gilthead sea bream Sparus aurata are in accordance with those of other carnivorous fishes and congruent to the feeding habits of the cage-reared fish.
References