Research Article
Effect of Environmental Climatic Conditions on levels of Some Hormones, Vitamins and Trace Elements in Blood and Seminal Plasma of Rabbits
Department of Biological Applications, Radioisotopes Applications Division, Nuclear Research Center, Atomic Energy Authority, Inshas, Cairo, Egypt
*Corresponding author: Alsaied Alnaimy Habeeb, Biological Applications Department, Radioisotopes Applications Division, Nuclear Research Centre, Atomic Energy Authority, Cairo, Egypt, Cell: 00201283912177 E-mail: dr_alnaimy@yahoo.com
Received: June 28, 2018 Accepted: July 15, 2018 Published: July 20, 2018
Citation: Habeeb AA, Sharoud MN, Basuony HA, Michael MI. Effect of Environmental Climatic Conditions on levels of Some Hormones, Vitamins and Trace Elements in Blood and Seminal Plasma of Rabbits. Int J Biotechnol Recent Adv. 2018; 1(1): 18-23. doi: 10.18689/ijbr-1000104
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The number of 30 male New Zealand White (NZW) rabbits was used in the present research. The rabbits exposed to two different climatic conditions. The first was under mild condition (MC) where the ambient temperature (AT) and relative humidity (RH%) values were 16.0oC and 72.5%, respectively, during January, February and March (10 weeks). The second was under hot condition (HC) where the AT and RH% values were 36.0oC and 62.5%, respectively, during June, July and August (10 weeks). Blood samples and semen by artificial vagina using a female teaser rabbit were collected from the bucks at two weeks intervals during the two experimental periods. The levels of testosterone and cortisol hormones and cyanocobalamin (B12) and folic acid (B9 ) vitamins, in plasma of the blood and in seminal plasma of semen were estimated by the radioimmunoassay technique. The tracer in the two hormones (testosterone and cortisol) was labelled with iodine-125 (125I) and the tracer in the two vitamins (B12&B9 ) was labelled with (57Co). Zink and selenium concentrations in plasma or in seminal plasma were analyzed by a single dilution micro method using Atomic Absorption Spectrophotometer. Plasma testosterone level in bucks was lower under hot summer season than that under mild climatic condition of winter either in blood or seminal plasma. Plasma cortisol level was higher under hot than under mild climate. Folic acid and cyanocobalamin concentrations as well as zinc and selenium concentrations in blood plasma and seminal plasma were lower significantly under hot conditions than that under mild conditions. It can be concluded that exposure rabbits to severe heat stress conditions during hot summer season of Egypt have adversely affects their hormones, vitamins and trace elements. The drastic changes that occur in biological functions in heat stressed rabbits like depression in feed intake and utilization as well as disturbances in water, protein, energy and mineral metabolism may be responsible in reducing the secretion of testosterone and increasing cortisol which responsible on that depression in plasma vitamins and trace elements in rabbits during summer season.
Keywords: Rabbit; Hormones; Vitamins; Trace elements; Blood; Seminal plasma
Introduction
Rabbits in Egypt suffer from heat stress during the long hot humid climate in summer (May to November). A temperature of 13-20° C is known as the comfort zone for rabbits but at higher temperature, the appetite depressed, the productive and reproductive performances are impaired and the resistance to disease is decreased [1, 2]. Rabbits above 35 ° C, can no larger regulate their internal temperature and heat prostration sets in [3]. Reproductive performance depends on the production of sexual hormones, especially, testosterone and may therefore be suppressed when stressors interfere with sexual hormones. Glucocorticosteroids are known to interfere with reproductive function and there is some evidence that acute stressors impair reproductive performance during critical periods of the reproductive cycle, early pregnancy and lactation [4].
Vitamin B9 prevent anemia during pregnancy and helps in the metabolism and synthesis of several amino acids which used in the metabolism of fats, muscular growth and immune system. Folate also helps pyridoxal phosphate enzyme to complete the metabolic reactions. In addition, folic acid is essential for cell division, production of DNA and RNA and assists the prevention of changes in DNA which may lead to cancer. Deficiency of B9 caused anemia, depression, dementia, increased rate of heart disease and cancer, loss of appetite and weight loss [5]. Vitamin B12 (cobalamin and cyanocobalamin) is an active coenzyme, vital for processing carbohydrates, proteins and fats and also helps oxidation of several compounds, so CoA and the Krebs cycle are dependent on B12 [6]. In addition, B12 helps nerve cells, red blood cells and the manufacturing and repair of DNA. Vitamins B12 also control the processes of tissue synthesis and aid in protecting the integrity of the cell’s plasma membrane and play a key role in several body functions, such as mitochondrial energy metabolism; energy releasing and haematopoietic function [7]. In addition, vitamin B12 helps nerve cells, red blood cells, and the manufacturing/repair of DNA. Vitamin B12 also is vital for processing carbohydrates, proteins and fats, which help make all of the blood cells in our bodies. Lack of B12 results in anemia, constipation, heart disease, depression, weakness, neurological failures, permanent nerve damage, nausea, flatulence, loss of appetite, confusion, weight loss, numbness/tingling in hands and feet, difficulty in maintaining balance, memory loss, and soreness of the mouth or tongue [8].
The maintenance of mineral balance in animals is of profound importance for their reproduction and milk production due to their role in bone and teeth formation, blood clotting, proper functioning of nerve tissue, regulation of osmotic pressure in body fluids, maintenance of homeostasis in the acid-balance and acting as co-factors of enzymes or as catalysts in enzymatic reaction [9]. Selenium was an integral part of the enzyme glutathione peroxidase which destroys lipid peroxides for protecting cell membranes against peroxidative damage and plays a role in the electron transport chain [10]. Zinc is an important essential nutrient that is required at every stage of the life cycle for normal growth, development, and function in all animal species due to its functions in a large number of zinc metallo enzymes [11].
The objective of this experiment was to study the effects heat stress conditions of summer season on hormones (testosterone and cortisol), vitamins (vit.B9 and vit.B12) and trace elements (zinc and selenium) in both blood and semen of New Zealand rabbit’s bucks.
Materials and Methods
The practical work and the biochemical analysis were carried out in Rabbits Farm, Project of Experimental Farms in Radioisotopes Application Division, Biological Applications Department, Nuclear Research Centre, Atomic Energy Authority, Inshas, Cairo, Egypt. This work was reviewed and approved by the Animal Care and Welfare Committee of Zagazig University, Egypt (ANWD-206).
Location
The experimental work was carried out in rabbits farm of Biological Application Department, Radioisotopes Applications Division, Nuclear Research Centre, Atomic Energy Authority, Inshas, Egypt (latitude 31° 12' N to 22 ° 2' N, longitude 25 ° 53' E to 35° 53' E).
Experimental procedure
The number of 30 New Zealand White (NZW) rabbit’s bucks was used in the present research. The rabbits in this study exposed to two conditions. The first was under mild condition (MC) where the ambient temperature (AT) and relative humidity (RH%)values were 16.0oC and 72.5%, respectively, during January, February and March (10 weeks) while the second was under hot condition (HC) where the AT and RH% values were 36.0oC and 62.5%, respectively, during June, July and August (10 weeks).
Animal housing and management
The animals were housed in a part of the Rabbitary building during winter and summer conditions. The Rabbitry building was naturally ventilated through wired windows and provided with automatic controlled sided exhaustion fans. The animals were individually housed in galvanized wired. The galvanized wire cage batteries were arranged in rows back to back. Urine and faces dropped from cages on the floor were cleaned daily. All animals were kept under the same managerial and hygienic conditions in each period. The rabbits in all groups were fed the same diet during winter and summer periods. The ingredients of the commercial pelted diet are 42.50% clover hay, 24.0% wheat bran, 15.0% yellow corn, 10% Soybean meal (44% CP), 5% molasses, 1.75% bone meal, 0.70% calcium carbonate, 0.55% sodium chloride, 0.35% Vitamins & minerals premix and 0.15%DL-Methionine. The chemical analysis are 18.00% crude protein., 2.8% ether extract, 12.0% crude fiber and 2600 kcal DE/kg diet according to AOAC [12]. Each kilogram of vitamin and minerals premix contained: 10.000 IU Vit. A, 900 IU Vit D3, 2mg Vit K, 50mg Vit E, 2mg Vit B1 , 6mg Vit B2, 2mg Vit B6, 0.01mg Vit. B12, 20mg panathonic acid, 50mg niacin, 5mg folic acid, 1.2 mg biotin, 12000mg choline, 3 mg copper, 0.2mg iodine, 75mg iron, 30mg manganese, 70mg zinc, 0.1mg selenium, 0.1mg cobalt and 0.04mg magnesium (Pfizer-Co., Egypt).
Meteorological data and temperature humidity index (THI) estimation
Air temperature (°C) and relative humidity (%) inside the rabbitary building were measured once a day weekly four times at 12.00, 13.00, 14.00 and 15.00 hours using automatic thermo-hygrometer and the averages were 16±1.0°C and 72.5 ±1.2% during Mild climate and 36.0±1.1°C and 62.5±1.4% during Hot climate, respectively. The temperature-humidity index (THI) was calculated using the equation as follows:
THI = db°C – [(0.31-0.31 RH) (db°C -14.4)] where db°C=dry bulb temperature in Celsius and RH= relative humidity percentage /100.
The THI values obtained were then classified as follow: <27.8= absence of heat stress, 27.8 to < 28.9= moderate heat stress, 28.9 to <30.0 = severe heat stress and 30.0 and more = very severe heat stress [4]. The estimated THI values were 17.68 during the mild and 31.01 in the hot period, indicating absence of heat stress in the first period and exposure of rabbits to very severe heat stress in the second one.
Estimation of vitamin, hormones and trace elements in blood and semen
Blood samples were collected from the bucks at two weeks intervals during experimental periods. The blood samples were collected from marginal ear vein in vacationer tubes and were centrifuged for 20 minutes at 2000 x g to obtain plasma which was kept in a refrigerator (-20) until blood vitamin, hormones and trace elements were analyses. Semen was collected at two week intervals from each buck (5 times during each of mild and hot period) by artificial vagina using a female teaser rabbit. The temperature of the inner rubber sleeve of the artificial vagina was adjusted to 41- 43°C and the lubrication of the inner sleeve was performed using white Vaseline.
The levels of B12, folic acid, testosterone and cortisol in both plasma and seminal plasma were estimated by the radioimmunoassay (RIA) technique using the coated tubes kits, Diagnostic Systems Laboratories, Inc. Webster, Texas, USA and counting in the Laboratory of Biological Applications Department, Atomic Energy Authority, using computerizes Gamma Counter. The tracer in the two vitamins was labelled with (57Co) and the tracer in the two hormones was labelled with iodine-125 (125I).
Zink and selenium concentrations in plasma and seminal plasma were analyzed by a single dilution micro methods using Perkin-Elmer Crop, model 290B Atomic Absorption Spectrophotometer, Norwalk, C.T. in the Laboratory of Soil and Water Department, Atomic Energy Authority.
Statistical Analysis
pThe data were statistically analyzed using computer system (SAS, 2013) by ANOVA according the following model Yik = µ + Ci + eik where, µ= the overall mean; Ci = the fixed effect of the Climatic periods) (1= MC and 2= HS; i= 1,2) and eik = random error. The percentage change due to heat stress was calculated as follows: {(mild-hot) x 100}/mild.Results and Discussion
Effects of heat stress conditions of hot summer season on testosterone hormonal level
Plasma and seminal plasma testosterone concentrations in rabbit’s bucks were lower under hot than that under mild climate after 2 to 10 weeks of hot exposure (Table 1). The overall mean of testosterone levels in blood plasma and seminal plasma under mild climate were 124.56 and 10.52 ng/ml and decreased significantly (P<0.05, P<0.01) under heat stress conditions to 110.18 and 6.96 ng/ml, respectively. The decrease percentages due to heat stress were ranged between 11-12% in blood plasma and 23- 40% in seminal plasma according to experimental week of heat stress exposure with overall mean of 12.0 and 34.0% in blood plasma and seminal plasma, respectively. Results in Table (1) showed also that testosterone level was not affected significantly due to week of exposure period (length exposure period from week 2 to week 10) in each of mild and hot climatic conditions.
With prolonged heat exposure, the hypothalamic hormone releasing factors are suppressed and consequently, the pituitary hormones and other hormones, either autonomous or pituitary controlled are decreased [14]. The hormonal concentrations are altered by high environmental temperature, particularly; the hormonal secretions are known to be of major importance in productive and reproductive performance which are thyroid hormones, male and female sex hormones, insulin and aldosterone [15].
In addition, the drastic changes that occur in biological functions in animals like depression in feed intake and utilization as well as disturbances in water, protein, energy and mineral metabolism may be responsible in reducing the secretion of testosterone.
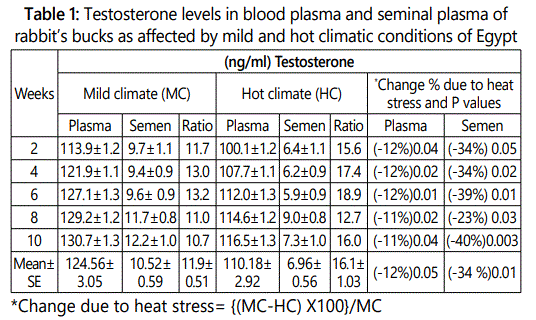
Testosterone plasma/semen ratio was 11.9 under mild climate and increased to 16.1 under heat stress conditions. This result indicated that testosterone level in plasma was 11.9 or 16.1 multiply with testosterone level in seminal plasma under mild or hot climatic conditions, respectively. This increase in testosterone plasma/seminal ratio attributed to that the percentage decreases due to heat stress in testosterone concentration in seminal plasma (12.0) was more than the percentage decrease in blood plasma (34%).
Effects of heat stress conditions of hot summer season on cortisol hormonal level
Plasma and seminal plasma of cortisol concentrations in rabbit’s bucks were higher under hot than that under mild climate after 2 to 10 weeks of hot exposure (Table 2). The overall mean of cortisol levels in blood plasma and seminal plasma under mild climate were 4.86 and 2.22 ng/ml and increased significantly (P<0.01) under heat stress conditions to 8.64 and 3.04 ng/ml, respectively. The increase percentage due to heat stress were ranged between 62-89% in blood plasma and 30-41% in seminal plasma according to week of heat stress exposure with overall mean of 78 and 37% in plasma and seminal plasma, respectively. Cortisol level was not affected significantly due to length exposure period of bucks to hot mild and hot climatic conditions. that under mild climate after 2 to 10 weeks of hot exposure (Table 2). The overall mean of cortisol levels in blood plasma and seminal plasma under mild climate were 4.86 and 2.22 ng/ml and increased significantly (P<0.01) under heat stress conditions to 8.64 and 3.04 ng/ml, respectively. The increase percentage due to heat stress were ranged between 62-89% in blood plasma and 30-41% in seminal plasma according to week of heat stress exposure with overall mean of 78 and 37% in plasma and seminal plasma, respectively. Cortisol level was not affected significantly due to length exposure period of bucks to hot mild and hot climatic conditions.
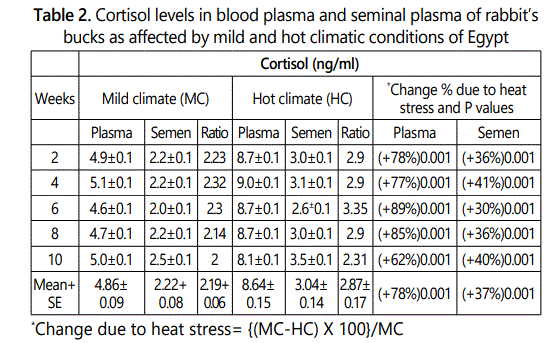
Cortisol plasma/semen ratio was 2.19 under mild climate and increased increased to 2.87 under heat stress conditions. This result indicated that cortisol level in plasma was nearly 2 or 3 multiply with cortisol level in seminal plasma under mild or hot climatic conditions, respectively. This increase in cortisol plasma/seminal ratio attributed to that the percentage decreases due to heat stress in cortisol concentration in seminal plasma (37%) was less than the percentage decrease in plasma (78%).
The increase in cortisol level which occurs during the chronic heat stress is attributed to the fact that cortisol is thermogenic in animals. Adrenocortical activity under thermal stress is a thermoregulatory protective mechanism preventing metabolic heat production in a hot environment for animal adaptation to heat stress conditions [14]. Activation of the hypothalamic pituitary adrenal axis and the consequent increase of plasma glucocorticoid concentrations are perhaps the most important responses of animals to stressful conditions. Adrenal corticoids, mainly cortisol, elicit physiological adjustments which enable animals to tolerate stressful conditions [16] [17]. Cortisol secretion from the adrenal gland through increase in ACTH secretion by the anterior pituitary gland often increases greatly in stressful situations [14]. Animals respond to stress by releasing ACTH from the anterior pituitary gland which causes the adrenal cortex to release aldosterone and corticosterone [18]. Activation of the hypothalamic pituitary adrenal axis and the consequent increase of plasma glucocorticoids concentrations are perhaps the most important responses of animals to stressful conditions. Adrenal corticoids, mainly cortisol, elicit physiological adjustments which enable animals to tolerate stressful conditions [19]. In addition, hormonal secretions are known to be of major importance in body thermoregulation which are cortisol and aldosterone [15]. The drastic changes that occur in biological functions in animals may be responsible in increasing the catabolic hormones like cortisol.
Effects heat stress conditions of summer season on vitamins B9 level
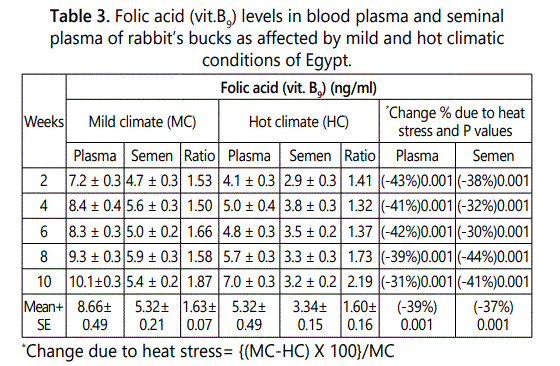
Folic acid (vit. B9 ) concentrations in plasma and seminal plasma of rabbit’s bucks were lower under hot than that under mild climate after 2 to 10 weeks of hot exposure (Table 3). The overall mean of vit. B9 levels in blood plasma and seminal plasma under mild climate were 8.66 and 5.32 ng/ml and decreased significantly (P<0.001) under heat stress conditions to 5.32 and 3.34 ng/ml, respectively. The percentages decrease in vit. B9 due to heat stress were ranged between 31 to 43% in blood plasma and 30 to 41% in seminal plasma according to week of heat stress exposure with overall mean of 39.0 and 37.0% in plasma and seminal plasma, respectively.
Folic acid plasma/semen ratio was 1.6 under each of mild and hot climates. This result indicated that Folic acid level in plasma was 1.6 multiply with Folic acid levels in seminal plasma under mild and hot climatic conditions (Table 3). However, vit. B9 level in rabbits was not affected by length of exposure period under mild or hot climatic conditions.
Effects heat stress conditions of summer season on vitamins B12 level
The overall mean of vit.B12 were 26.92 and 22.1 pg/ml under mild and hot climates, respectively. These results indicated that vitamin B12 concentrations decreased by more than 18% of its level due to exposure the rabbits to the stressful conditions of summer season of Egypt. Vit. B12 concentration in seminal plasma of rabbit’s bucks was lower under hot than that under mild climate. The overall mean of vit. B12 level in seminal plasma under mild climate was 20.94 pg/ml and decreased significantly (P<0.05) under heat stress conditions to 17.84 pg/ml.
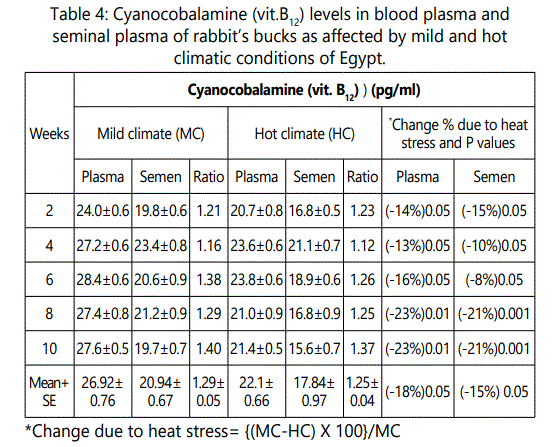
The decrease percentage due to heat stress was ranged between 8 to 21% in seminal plasma according to week of heat stress exposure with overall mean of 15.0%. However, vit. B12 level was not affected by exposure weeks either under mild or under hot climatic conditions. Vit. B12 in plasma/semen ratio was nearly equal 1.3 under each of mild and hot climates. Vit. B12 level in plasma was 1.3 multiply with vit. B12 level in seminal plasma under the tow conditions (Table 4).
In this respect, [20] found that vitamin B12 concentrations decreased by more than 25% of its level due to exposure the rabbits to the stressful conditions of summer season of Egypt.
Effects heat stress conditions of summer season on zinc concentrations
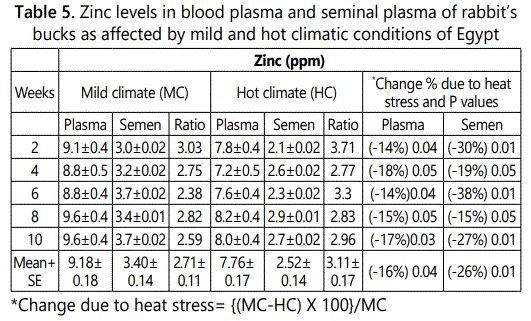
Zinc concentrations in plasma and seminal plasma of rabbit’s bucks were lower under hot than that under mild climate after 2 to 10 weeks of hot exposure (Table 5). The overall mean of zinc levels in blood plasma and seminal plasma under mild climate were 9.18 and 3.40 ppm and decreased significantly (P<0.05; P<0.01) under heat stress conditions to 7.76 and 2.52 ppm, respectively. The decrease percentage due to heat stress were ranged between 14 to 18% in blood plasma and between 15 to 38% in seminal plasma according to week of heat stress exposure with overall mean of 16.0 and 26.0% in plasma and seminal plasma, respectively. Zinc plasma / semen ratio was 2.7 and 3.1 under mild and hot climates, respectively. This result indicated that zinc level in plasma was 2.7 multiply with zinc level under mild climate and 3.1 multiply with zinc level under hot climate. Zinc is an important essential nutrient that is required at every stage of the life cycle and its functions in large number of zinc metallo-enzymes and also is required for normal growth, development and function in all animals [21]. Therefore, lesser degrees of deficiency are most pronounced during periods of rapid growth and in those cells or tissues that either turn over or grow most rapidly. Characteristics of deficiency include growth retardation, delayed sexual maturation, skeletal abnormalities and impaired reproduction in both males and females [21].
Effects heat stress conditions of summer season on selenium concentrations
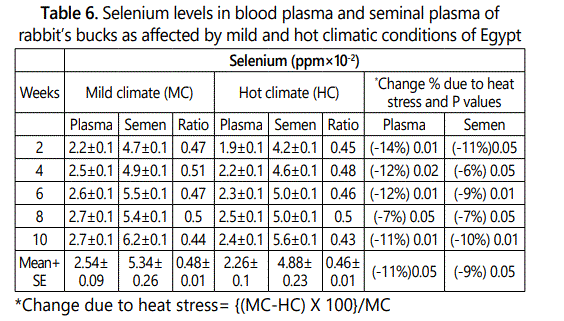
Selenium concentrations in plasma and seminal plasma of rabbit’s bucks were lower under hot than that under mild climate after 2 to 10 weeks of hot exposure (Table 6). The overall mean of selenium levels in blood plasma and seminal plasma under mild climate were 2.54 and 5.34 ppm×10-2 and decreased significantly (P<0.05) under heat stress conditions to 2.26 and 4.88 ppm×10-2, respectively. The decrease percentage due to heat stress were ranged between 7 to 14% in blood plasma and between 6 to 11% in seminal plasma according to week of heat stress exposure with overall mean of 11 and 9.0% in plasma and seminal plasma, respectively. Selenium plasma/semen ratio was 0.48 and 0.46 under mild and hot climates, respectively. This result indicated that selenium level in plasma was 0.47 multiply with selenium level in seminal plasma under any conditions.
Selenium is an integral part of the enzyme glutathione peroxidase which converts hydrogen peroxide to water and is an important component of the cellular antioxidant system [9]. The glutathione peroxidase enzyme is also related to functions the cytosol of the cell; protects polymorphonuclear neutrophils against reactive oxygen species production, increases survival of leukocytes involved in cellular defence and improves the activity of these cells in the mammary gland [22]. The positive role of selenium in the immune system is well documented by [23]. Selenium was identified as a component of type I iodothyronine5?-deiodinase enzyme that converts T4 to T3 [10]. Several selenoproteins have been identified with functions connected to the thyroid hormone metabolism, testes and sperm function and muscle metabolism [9].
Concentrations of selenium in serum and whole blood have been used as an index of selenium status because, in general, increased concentrations of selenium in serum or whole blood have been related to reduced mastitis and improved neutrophil function [24]. A lack of selenium lowers the production of leucotrienes of polymorphonuclear leukocytes and thus lowers chemotaxis of neutrophils [25]. The concentration of selenium in milk is increased when cows are fed additional selenium. Selenium concentration of milk ranges from 0.01 to 0.025 mg/kg and based on the effect of selenium on mastitis, concentrations of selenium in whole blood should be greater than about 0.18 g/ml or approximately 0.08 g/ml for plasma [26].
Conclusion
It can be concluded that exposure of rabbits to < 30 THI (temperature-humidity index) units or more as severe heat stress during summer, adversely affects their hormones, vitamins and trace elements. The drastic changes that occur in biological functions in rabbits like depression in feed intake and utilization as well as disturbances in water, protein, energy and mineral metabolism may be responsible in reducing the secretion of testosterone and increasing the catabolic hormones like cortisol which responsible on that depression in plasma vitamins and trace elements of rabbits during summer season. It is also concluded that testosterone, cortisol, vit.B9 , vit. B12 and zinc concentrations in blood plasma of rabbit’s bucks were higher than that concentrations in seminal plasma by 12-16, 2-3, 1.6, 1.3 and 2.7-3.1 times. On the other hand, selenium in seminal plasma was higher than that concentration in blood plasma by 2 times, indicating the importance of selenium in spermatogenesis process in the testes.
References
- Marai IFM, Habeeb AAM, Gad AE. Reproductive traits of male rabbits as affected by climatic conditions, in the subtropical environment of Egypt. Animal Science. 2002b; 75: 451- 458.doi: 10.1017/S1357729800054394
- Marai IFM, Habeeb AAM, Gad AE. Performance of New Zealand White and Californian male weaned rabbits in the subtropical environment of Egypt. Animal science Journal. 2008; 79(4): 472-480. doi: 10.1111/j.1740-0929.2008.0052.x
- Marai IFM, Habeeb AAM. Thermoregulation in Rabbits. Proceeding of the 1st international conference on “Rabbits Production in Hot Climates”. Zagazig University, Egypt, 1994; 8: 33-41.
- Marai, IFM, Habeeb AAM, Gad AE. Rabbits productive, reproductive and physiological performance traits as affected by heat stress a review. Livestock Production Science. 2002a; 78: 71- 90.
- Bender DA. Nutritional biochemistry of the vitamins. Second Edition, New York: Cambridge University Press. 1992; 269- 317.
- Lebas F. Vitamins in rabbit nutrition, Literature Review and Recommendations. World Rabbit Science. 2000; 8(4): 185-192. doi: 10.4995/wrs.2000.438
- Ellenbogen L, Cooper BA. Vitamin B12. In: Machlin LJ. ed. Handbook of vitamins, 2nd edition. Marcel Dekker pp. New York. 1991; 491-536.
- Petchkrua W, Little JW, Burns SP, Stiens SA, James JJ. A retrospective study: Modem Nutrition in Health and Disease, 8th ed. Philadelphia. Lea and Febiger. 1994; 426-431.
- Habeeb AAM, Teama FEI. Influence of deficiency or supplementary selenium and ά-tochopherol (vitamin E) in the diet of pubertal male zaraibi goats on fertility, semen quality and testicular traits. Isotope and Radiation Research. 2013; 45(2): 33-348.
- Habeeb AAM, Teama FEI, EL-Tarabany AA. Effect of adding selenium and vitamin E to the diet on reproductive traits of female zaraibi goats and growth of their kids. Isotope and Radiation Research. 2012; 44(3): 693-709
- Habeeb AAM, EL-Tarbany AA, GadAE. Effect of zinc levels in diet of goats on reproductive efficiency, hormonal levels, milk yield and growth aspects of their kids. Global Veterinaria, 2013; 10(5): 556-564. doi: 10.5829/idosi. gv.2013.10.5.7367
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis, Washington, DC. 1990.
- SAS Institute Inc. Statistics Analysis System User’s Guide. SAS/STAT Procedures Guide for Personal Computer. Ed 9.2. Cary, North Carolina, USA. 2008.
- Habeeb AAM. Marai IFM, Kamal TH. Heat Stress. In: Farm Animals and the Environment. Commonwealth Agricultural Perau International. UK. 1992; 27-47.
- Marai IFM, Habeeb AAM. Managemental practices to ameliorate heat stress. International Conference on Animal, Poultry & Rabbit Production & Health. Institute Of Efficient Productivity, Zagazig University, Zagazig, Egypt. 1997: 179-183.
- Boiti C, Chiericato GM, Filotton N, Conali C. Effects of high environmental temperature on plasma testosterone, Cortisol, T3 and T4 levels in the growing rabbit. Journal of Applied Rabbit Research, 1992; 15: 447-455.
- Okab AB, El-Banna SG, Koriem AA. Influence of Environmental Temperatures on Some Physiological and Biochemical Parameters of Male New-Zealand Rabbits. Slovak J Anim Sci. 2008; 41: 12-19.
- Marai IFM, Habeeb AAM. Review article: Buffalo’s biological functions as affected by heat stress. Livestock Science, 2010; 127: 89-109. doi: 10.1016/j. livsci.2009.08.001
- Christison GI, Johnson HD. Cortisol turnover in heat-stressed cows. J. Anim. Sci. 1972; 53: 1005-1010.
- Habeeb AAM, Elwan KM, Marai IFM, EL-Drawany AA, EL-Tarabany AA, et al. Effect of amelioration summer heat stress condition techniques on some blood hormones, vitamins and trace elements in rabbit bucks. Isotope and Radiation Research. 2010; 42: 4: 1353-1373.
- Habeeb AAM, EL-Tarbany AA, GadAE. Effect of zinc levels in diet of goats on reproductive efficiency, hormonal levels, milk yield and growth aspects of their kids. Global Veterinaria, 2013; 10(5): 556-564.
- Smith KL, Hogan JS, Weiss WP. Dietary vitamin E and selenium affect mastitis and milk quality. J. Anim. Sci. 1997; 75: 1659-1665.
- McKenzie RC, Rafferty TS, Beckett GJ. Selenium: an essential element for immune function. Immunol Today. 1998; 19(8): 342-345.
- Habeeb AAM, Elwan KM, Marai IFM, EL-Drawany AA, EL-Tarabany AA, et al. Effect of amelioration summer heat stress condition techniques on some blood hormones, vitamins and trace elements in rabbit bucks. Isotope and Radiation Research. 2010; 42: 4: 1353-1373.
- Habeeb AAM, Elwan KM, Marai IFM, EL-Drawany AA, EL-Tarabany AA, et al. New Techniques for improving the Rabbit’s Semen Properties under Hot Summer Conditions. Egyptian J. of Applied Sciences. 2011; 26(3): 1-24.
- Jukola E, Hakkarainen J, Saloniemi H, Sankari S. Blood Selenium, Vitamin E, Vitamin A, and ß-Carotene Concentrations and Udder Health, Fertility Treatments, and Fertility. J. Dairy Sci. 1996; 79(5): 838- 845.


