Research Article
One-step Card Tests for Fecal Calprotectin and Lactoferrin in Patients with Inflammatory Bowel Disease
Department of Clinical Immunology, University Hospital Lozenetz, Sofia, Bulgaria
*Corresponding author: Tsvetelina Velikova, Department of Clinical Immunology, University Hospital Lozenets, Kozyak 1 str1407, Sofia, Bulgaria, Cell: 00359883306049 E-mail: tsvelikova@medfac.mu-sofia.bg
Received: June 28, 2018 Accepted: July 7, 2018 Published: July 12, 2018
Citation: Velikova TV, Altankova I. One-step Card Tests for Fecal Calprotectin and Lactoferrin in Patients with Inflammatory Bowel Disease. Int J Biotechnol Recent Adv. 2018; 1(1): 12-17. doi: 10.18689/ijbr-1000103
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective
The diagnosis of inflammatory bowel disease (IBD), as well as the evaluation of disease activity, can be challenging. The aim of the study was to evaluate the significance of onestep card tests for fecal calprotectin (FC) and fecal lactoferrin (FL) in patients with IBD.
Material and methods: We have examined fecal samples for FC and FL obtained from 29 patients with IBD (15 with ulcerative colitis - UC and 14 with Crohn’s disease - CD) and 12 healthy individuals. A qualitative one-step card test was used for each marker.
Results: We obtained a sensitivity of 72.4% and 44.8%, for FC and FL, respectively, and positive predictive value (PPV) of 100% for both tests, and negative predictive value (NPV) 60% and 42%, respectively. We found a correlation between the disease activity of CD patients and FC level (p = 0.024, r = 0.583) with Likelihood Ratio (LR) 4.31 (p = 0.038). FC demonstrated also association with platelet count (p = 0.007), serum iron (p = 0.031) and disease duration (p = 0.027), whereas FL showed association with platelet count (p = 0.001), CRP (p = 0.023) and presence of complications (p = 0.017).
Conclusion: The one-step card test for fecal markers is useful in the diagnosis of IBD, although FC showed better performance than FL. However, both markers are useful in clinical practice where FC can assess the severity of CD, while FL is elevated in complicated IBD.
Keywords: Fecal markers; Fecal calprotectin; Fecal lactoferrin; One-step card test; Intestinal inflammation; Mucosal inflammation; Inflammatory bowel disease; Ulcerative colitis; Crohn’s disease
Introduction
Any inflammation, including in chronic inflammatory bowel disease (IBD), begins as acute inflammation which regardless the underlying causes two major effects on microcirculation - exudation of fluids and extravasation of polymorphonuclear cells (mainly neutrophils) [1]. In the presence of upregulated adhesion molecules on endothelial cells, the chance that inflammatory cells will migrate to the sites of local intestinal inflammation increases. Once in the tissues, neutrophils begin to perform phagocytosis accompanied by marked oxygen consumption in a process called a “respiratory burst”. Typically, acute inflammation subsides within hours to days as well as the neutrophils are living [1]. Neutrophils are a major source of both calprotectin and lactoferrin - cytosolic proteins that are secreted in acute inflammation. During intestinal inflammation, both markers could be found into the lumen of the gastrointestinal tract. Fecal calprotectin has a bacteriostatic and fungistatic effect by inhibiting the binding and internalization of bacterial agents [2], and lactoferrin acts toxic directly on microorganisms [3]. Fecal biomarkers for the evaluation of intestinal inflammation are increasingly used in gastroenterological practice in terms of their bowel specificity. Such markers associated with inflammation are calprotectin (FC), lactoferrin (FL), fecal S100A12, lysozyme, leukocyte esterase, elastase, and others [4].
Calprotectin was described by Fagerhol et al. in 1979 as a heterodimeric protein composed of two small anionic proteins (MRP8 and MRP14) belonging to the family of calcium-binding proteins [5]. Calprotectin accounts for up to 60% of neutrophil cytosolic proteins, with low concentrations found in both monocytes and activated macrophages, and absent in platelets and lymphocytes [6]. It is expressed on the surface of the cells or inside the squamous epithelium of the mucosa and the skin. Its bacteriostatic and fungistatic effects are associated with inhibiting the penetration of bacterial agents [5]. Epithelial cell lines that consistently express calprotectin are less susceptible to pathogens such as Listeria and Salmonella [6]. The determination of FC reflects indirectly the activation of granulocytes and their influx into the intestinal lumen [7]. The study of the FC allows diagnosing Crohn’s disease (CD), especially when the affected area of the gastrointestinal tract is unapproachable for endoscopic examination [8].
Another fecal marker useful in IBD management is fecal lactoferrin (FL). Lactoferrin is a protein belonging to the transferrin family - glycoproteins that bind iron. It consists of a single polypeptide chain with a molecular weight of 78 kDa, secreted into the secondary granules of neutrophils [3]. It is found in almost all excretions - tears, nasal secretions, saliva, intestinal mucus and genital secretions. Lactoferrin is a significant component of the first line of protection in mammalians and its expression is increased in the respiratory or gastrointestinal systems in response to inflammatory stimuli or allergens [3]. Lactoferrin is a multifunctional protein with many physiological functions such as promoting the absorption of iron in the intestines and the growth of intestinal cells, possessing anti-inflammatory properties in the gastrointestinal tract and direct toxicity to microbial agents, regulation of myelopoiesis, stimulating the proliferation and differentiation of osteoblasts and inhibiting osteoclasts [9]. It is generally accepted that FL, similar to FC, reflects the activity of neutrophils and is a useful non-invasive marker of intestinal inflammation in IBD patients [10]. Commercial ELISA kits for FC and FL determination in feces are available, but there are also rapid tests [11, 12].
The aim of the present study was to compare PK and FL, tested with a test-plate method, as indicators of intestinal inflammation.
Materials and methods
Subjects of the study
The study included 29 individuals with IBD clinically, histologically and endoscopically proven diagnosis according to the ECCO Consensus Diagnostic Criteria for BC (2010) and EC (2012) A control group of 25 persons without IBD and no data on other intestinal or extracurricular severe accompanying diseases were also recruited. Fifteen patients were with ulcerative colitis, UC, and fourteen - with CD. The mean age of the patients was 41 ± 4 years and the male:female ratio was 1:1. The overwhelming proportion of the patients (78.7%) was at a stage of activity, assessed by Crohn`s disease activity index, CDAI, for CD patients, and Mayo score for UC patients). The newly diagnosed were 23.4% of the IBD patients. No complications were observed in 62% of the patients, whereas in 38% of them was described at least one complication (i.e., fistulae, structures, etc.).
Specimen collection and preparation
The fecal samples were collected in chemically pure containers, and stored at -20° C according to the requirements of good laboratory practice and the commercial kit instructions. Before being tested, samples were thawed completely at room temperature.
Immunological testing
FC was determined by a ready-to-use One-Step Card CerTest Calprotectin (CerTest Biotec, Spain) and FL - by OneStep Card CerTest Lactoferrin (CerTest Biotec, Spain). Determining FC and FL by one-step card test is a rapid qualitative method with low complexity allowing measurement of a single sample fast and on the patient bench side. The method is a one-color colorimetric assay with a test plate preloaded with antibodies against human FC or FL, respectively (Figures 1a and 1b).
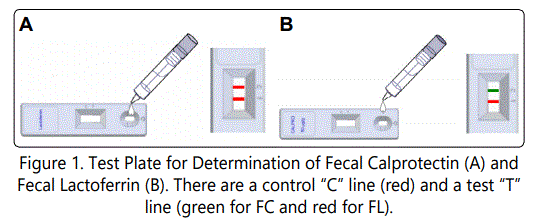
The tests were carried out according to the manufacturer’s instructions. A right extendable sensitivity of the two tests was 50 mg/kg. If a strip is not displayed in the T region, the test is negative.
Statistical analysis
We statistically analysed the row data using parametric and non-parametric tests (Software Package for Statistical Analysis SPSS®, v.19 (IBM). In addition, differences were considered statistically significant at p < 0.05.
Results
FC and FL were determined in stool samples of 29 patients with IBD (Table. 1).
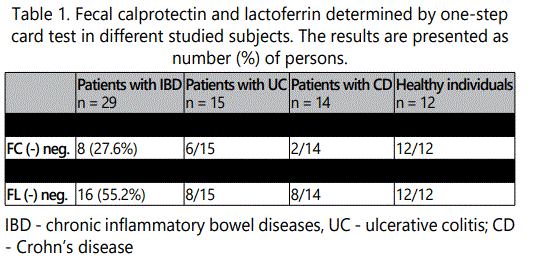
We did not find a positive FC result in any of the healthy individuals, whereas positive for FC were found 21 out of 29 patients with IBDD (72.4%). Among them, 12/14 of patients with CD were positive for FC, compared to 9/15 of patients with UC. Less than a half of the patients with IBD were positive for FL - 13 out of 29 (44.8%). None of the healthy individuals were positive for FL. The diagnostic sensitivity and specificity of the FC and FL, as well as the positive predictive value (PPV) and negative predictive value (NPV), calculated according to the corresponding formulas, are presented in Table 2.
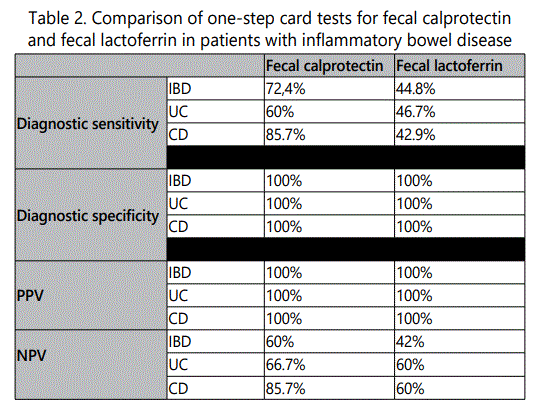
The diagnostic specificity of the FC was 100% due to the fact that none of the healthy individuals were positive for FC. The diagnostic sensitivity of the test for determining the patients with UC was 60%, for the patients with CD it was higher - 85.7% respectively, and for IBD was 72.4%. PPV of 100% indicated the high probability for IBD diagnosis in patients who have shown a positive result for FC. NPV of 60% for IBD indicated that the negative result did not exclude the diagnosis of IBD. NPV of FC was higher for CD patients. This indicated that a negative result in an individual most likely excluded the possibility of a patient having CD.
The diagnostic specificity of the FL was 100%. Diagnostic sensitivity for IBD patients, as well as for patients with UC and CD, was low - less than 50%. The PPV value of 100% indicated high ability patients with IBD and positive result for FL score to have this disease. The low NPV of 42% for IBD indicated that the negative result did not exclude the diagnosis of IBD. This value was 60% for UC and CD.
The diagnostic sensitivity of FC was shown to be significantly higher than that of FL, for IBD, as well as for UC and CD. Both FC and FL showed specificity and PPV of 100%. NPV value was lower for FL, indicating the weakness of the marker for IBD diagnosis exclusion when the result is negative. Regarding the clinical significance of FC and FL, we performed statistical analyzes for the presence of associations between the fecal markers and some laboratory parameters, clinical characteristics of the patients, as well as the activity of the diseases assessed by the corresponding Mayo score and CDAI indexes.
We have had a significant relationship between the duration of the disease in years and the presence of FC (r = 0.369, p = 0.027). Patients positive for FC had a longer duration of the disease than those who are negative for FC. The observed correlation was evaluated as mild to moderate (correlation coefficient r = 0.369). We also found that the presence of complications (intestinal and/or extraterrestrial) correlates moderately (r = 0.395) with the FC levels - patients who were positive for FC showed more frequent complications. We also obtained a significant association between FC levels and the type of treatment with the highest levels of FC observed in patients without therapy (p < 0.01).
We documented an association between FC levels and the following laboratory parameters in IBD patients: C - reactive protein, hemoglobin, ESR, albumin, leukocyte count, platelets count, and serum iron. Elevated FC levels correlated significantly with increased platelet counts in patients with IBD, as well as in patients with UC and CD. Elevated levels of FC showed an inverse correlation with the serum iron level (p <0.05), and a correlation with the ESR (p <0.05). Associations between FL levels and the duration of the disease (in years) (r = 0.553), the presence of complications, and the therapy being conducted at the time of collection of the biological material were observed. FL-positive patients also had higher C-reactive protein (p = 0.023), hemoglobin (p = 0.056) and platelet counts (p = 0.001) compared to patients who were negative for FL.
We found no significant relationship between the disease activity, both in the UC and CD, and the FL levels .The calculated Likelihood ratio (LR) = 2.73 (p> 0.05) for UC, as well as LR = 0.071 (p> 0.05) was also not statistically significant. An association with disease activity was observed only for FC in CD patients (r = 0.583, p = 0.024; R 4.319, p = 0.024).
The two quality tests for FC and FC showed congruent results which correlated moderately (Cramer’s coeff - 0.552, p <0.05).
Discussion
In order to reduce the time for diagnosing IBD and reduce the number of invasive studies, research has been urged to seeking simple, non-invasive, fast, cheap, and reliable methods of assessing intestinal inflammation and mucosal recovery after successful therapy. Of the markers studied in recent years, the most promising were the FC and FL derivated from the neutrophils [13]. The intestinal presence of fecal markers of inflammation is readily available and reliable because the intestinal contents are in constant and close contact with the intestinal walls where the neutrophils are. If the mucosa is inflamed, FC, FL and other inflammatory cell products can easily pass from the mucosa to the intestinal contents and could be measured in stool samples [13]. In practice, simpler, faster and easier methods for identifying both FC and FL are being introduced [14].
Meta analyzes from different studies documented average values of 89% (70-100%) for PPV and 81% (51-91%) for NPV in diagnosis of IBD patients [15-23]. Our results for PPV and NPV of FC and FL fit within the leading world research. Moreover, our results, consistent with those in the literature, showed that FC was a sensitive, but not completely specific marker of intestinal inflammation. FC is not a disease-specific indicator because an increase can be observed in any gastrointestinal state where neutrophils are abundant in the intestinal mucosa. However, it remains the best marker for the detection of inflammation in the gastrointestinal tract up to date.
We found significantly higher FC levels in IBD patients without therapy (p <0.01). In our study, we obtained a significant correlation between FC levels and platelet counts. Numerous authors report such correlation regardless of the duration or form of the disease [21]. A possible explanation lies in the recently published data on various hemostasis disorders in patients with inflammatory conditions. This raises the issue of underestimating the platelet count as a useful marker of active inflammation [21].
FC levels showed a correlation with serum iron (p <0.05) and ESR (p <0.019). We did not observe a correlation between FC levels and the serum marker for inflammation C- reactive protein and the leukocyte count in IBD patients, unlike other authors who establish such correlation [20-23].
Most authors have established correlations between FC levels and activity in UC and CD patients [25,26]. We found increased FC in patients with active disease, calculated by the respective scores. For both forms of IBD, FC was positive for the majority of the patients with disease activity. All patients with remission were found negative for FC, whereas 33% of the CD patients in remission were positive for the FC. All CD patients in activity were positive for FC, but one-third of the patients with UC and active disease were found negative for FC. These versatile data showed us that FC can be positive for patients both in activity and in remission, but it may also be negative in both cases, which should be considered. A significant correlation between FC levels and disease activity was found only for CD patients (p = 0.024 and LR = 4.319, p = 0.038), which suggested that the positive outcome for FC correlated with great certainty with disease activity. For patients with UC, we did not find a correlation between FC and disease activity, as well as Kolho et al., did not report such observations [11].
Lactoferrin is another protein released by neutrophils that can be found in the intestinal contents. FL is less studied than the FC. According to the literature, the sensitivity of FL for IBD is between 67-87%, the specificity - between 85-100%, PPV - 87-100%, NPV - 77-87% [10,13,18]. It is noteworthy the lower sensitivity we have received for patients with UC and CD (less than 50%). Probably this is related to the fact that we used a quality one-step card test method, and the published results were obtained mainly by ELISA methods. In spite of the negative results for FL, FL may be useful in the detection of intestinal inflammation. Furthermore, we found some associations of FL with clinical and laboratory results, as well as the activity of IBD patients. We confirmed that the presence of complications correlated moderately with the presence of FL in the feces (r = 0.553), i.e. patients with higher levels of FL had more frequent complications. A positive significant correlation was found between PF and C-reactive protein levels (p <0.05), hemoglobin (p = 0.054) and platelet count (p <0.01). In the literature, there is scarce data only for correlations between FL and CRP [21,23].
When we divided the patients with UC and CD into those with activity and those with remission, we again received mixed data on the FL positivity, similar to the data for FC. In both diseases at active state only about half of the patients were positive for FL. All patients with UC who were in remission were negative for FL, whereas 33% of CD patients in remission were positive for FL. Several studies have shown a significantly higher FL in an active disease than in remission [10,26-28]. According to these researchers, FL can be used to measure disease activity in IBD patients as well as to predict exacerbations. We believe that the observed discrepancy with our results may be related to the relatively small number of IBD patients studied and the different methods for detection of the fecal markers.
Since FL was a less studied marker, we compared its clinical significance with the more well-established FC. Diagnostic sensitivity of FC was shown as significantly higher than that of FL, both for IBD and for distinct UC and CD. Both tests showed specificity and PPV of 100%. NPV values were lower for FL, indicating the weakness of FL for exclusion IBD diagnosis in test subjects.
However, the two markers FC and FL correlated moderately in our study (Cramer’s coeff 0.552, p <0.05), which was also found by two other groups of investigators who did similar comparative studies on the two fecal markers [22,29,30].
Based on our comparative studies, the FC test showed better performance than the FL test. The publications comparing the two markers are scarce and often controversial, with conflicting results. Some studies conclude that the two fecal markers are equivalent in their ability to detect intestinal inflammation [18,19], while other studies, as well as ours, consider FC to be superior to FL [31]. Only a few studies demonstrate the greater accuracy of FL in distinguishing IBD patients from healthy subjects [32]. An interesting study by Langhorst et al. revealed a greater sensitivity of FC for detecting CD patients (81.4%), while FL - greater sensitivity (83.3%) for UC patients [30]. We found a higher sensitivity of FC to determine the patients with both diseases – UC and CD, compared to FL. In the literature, there is evidence of the importance of FL in distinguishing patients with active IBD from those with irritable bowel syndrome, IBS [33]. In IBS, LF levels are close to those of healthy controls, and in case of gut infections, they are moderately elevated [34]. Furthermore, FL is involved in the modulation of mucosal intestinal inflammation, as well as other not yet fully understood immunoregulatory functions [35].
This study has some limitations. The small size of the study population might have failed to detect substantial alterations of the results. However, it needs to be stressed that the results of this study corroborated that of other recent studies, and further follow-up of the study cohort is necessary to assess any long-term usefulness of these methods.
Conclusion
In conclusion, we can summarize that FC and FL are relatively well-documented biomarkers of neutrophil inflammation of the intestines. They are suitable screening markers for identifying patients to be directed to a subsequent endoscopy for further evaluation of the disease. FC and FL correlate with disease activity in UC and CD, as well as they are promising indicators for mucosal healing and monitoring the effect of the applied therapy.
Based on our studies, FC showed better characteristics than FL, but both tests correlated with different clinical and laboratory parameters and can be used in a combination for a better description of the patients.
References
- McDonald T. Immunology and Diseases of the Gut. 2006.
- Hestvik E, Tumwine JK, Tylleskar T, Grahnquist L, Ndeezi G, et al. Fecal calprotectin concentrations in apparently healthy children aged 0-12 years in urban Kampala, Uganda: a community-based survey. BMC Pediatrics. 2011. doi: 10.1186/1471-2431-11-9
- Connects O. Anti inflammatory activities of lactoferrin. Journal of the American College of Nutrition. 2001; 20 (5): 396S-397S. doi: 10.1080/07315724.2001.10719173
- Desai D, Faubion WA, Sandborn WJ. Review article: Biological activity markers in inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2007; 25(3): 247-255. doi: 10.1111/j.1365-2036.2006.03184.x
- Fagerhol MK. Calprotectin, a fecal marker of organic gastrointestinal abnormality. Lancet. 2000; 356 (9244): 1783-1784. doi: 10.1016/S0140-6736(00)03224-4
- Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infection and immunity. 2001; 69 (7): 4242-4247. doi: 10.1128/IAI.69.7.4242-4247.2001
- Roseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of the disease activity in ulcerative colitis by fecal calprotectin, and novel granulocyte marker protein. Digestion. 1997; 58 (2): 176-180. doi: 10.1159/000201441
- Gaya DR, Lyon TD, Duncan A, Neilly JB, Han S, et al. Fecal calprotectin in the evaluation of Crohn’s disease activity. Qjm. 2005; 98 (6): 435-441. doi: 10.1093/qjmed/hci069
- Naot D, Chhana A, Matthews BG, Callon KE, Tong PC, et al. Molecular mechanisms involved in the mitogenic effect of lactoferrin in Bone. 2011; 49 (2): 217-224. doi: 10.1016/j.bone.2011.04.002
- Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. The American Journal of Gastroenterology. 2003; 98 (6): 1309-1314. doi: 10.1111/j.1572-0241.2003.07458.x
- Kolho KL, Turner D, Veereman-Wauters G, Sladek M, de Ridder L, et al. Rapid test for fecal calprotectin levels in children with Crohn disease. Journal of pediatric gastroenterology and nutrition. 2012; 55 (4): 436-439. doi: 10.1097/MPG.0b013e318253cff1
- Schoepfer AM, Trummler M, Seeholzer P, Criblez DH, Seibold F. Accuracy of four fecal assays in the diagnosis of colitis. Diseases of the colon and rectum. 2007; 50 (10): 1697-1706. doi: 10.1007/s10350-007-0303-9
- Sipponen T. Diagnostics and prognostics of inflammatory bowel disease with fecal neutrophil-derived biomarkers of calprotectin and lactoferrin. Dig Dis. 2013; 31 (3-4): 336-344. doi: 10.1159/000354689
- Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, et al. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008; 46 (9): 1275-1280. doi: 10.1515/CCLM.2008.246
- Arijs I, De Hertogh G, Machiels K, Van Steen K, Lemaire K, et al. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with IBD before and after infliximab treatment. The American Journal of Gastroenterology. 2011; 106 (4): 748-761. doi: 10.1038/ajg.2011.27
- Atarashi K, Tanoue T, Umesaki Y, Honda K. Regulation of Th17 cell differentiation by intestinal commensal bacteria. Beneficiary microbes. 2010; 1(4): 327-334. doi: 10.3920/BM2010.0026
- Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, et al. Role of fecal calprotectin as a non-invasive marker of intestinal in ammation. Dig Liver Dis. 2003; 35(9): 642-647. doi: 10.1016/S1590-8658(03)00381-5
- D’Inca R, Dal Pont E, Di Leo V, Ferronato A, Fries W, et al. Calprotectin and lactoferrin in the evaluation of intestinal i and organic disease. International journal of colorectal disease. 2007; 22 (4): 429-437. doi: 10.1007/s00384-006-0159-9
- Schroder O, Naumann M, Shastri Y, Povse N, Stein J. Prospective evaluation of fecal neutrophil-derived proteins in the identification of intestinal inflammation: the combination of parameters does not improve the diagnostic accuracy of calprotectin. Alimentary pharmacology & therapeutics. 2007; 26 (7): 1035-1042. doi: 10.1111/j.1365-2036.2007.03457.x
- Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, et al. A simple method for the evaluation of intestinal inflammation in Crohn’s disease. Gut. 2000; 47 (4): 506-513. 10.1136/gut.47.4.506
- Eder P, Stawczyk-Eder K, Krela-Kazmierczak I, Linke K. Clinical utility of the evaluation of fecal calprotectin in Lesniowski-Crohn’s disease. Polskie Archiwum Medycyny Wewnetrznej. 2008; 118 (11): 622-626.
- Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, et al. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflammatory bowel diseases. 2009; 15(12): 1851-1858. doi: 10.1002/ibd.20986
- Vieira A, Fang CB, Rolim EG, Klug WA, Steinwurz F, et al. Fatal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC research notes. 2009; 2: 221. doi: 10.1186/1756-0500-2-221
- Walkiewicz D, Werlin SL, Fish D, Scanlon M, Hanaway P, et al. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflammatory bowel diseases. 2008; 14 (5): 669-673. doi: 10.1002/ibd.20376
- D’Haens G, Ferrante M, Vermeire S, Baert F, Noman M, et al. Fecal calprotectin is a surrogate marker Download for endoscopic lesions in inflammatory bowel disease. Inflammatory bowel diseases. 2012; 18 (12): 2218-2224. doi: 10.1002/ibd.22917
- Gisbert JP, Bermejo F, Perez-Calle JL, Taxonera C, Vera I, et al. Fecal calprotectin and lactoferrin for the prediction of in ammatory bowel disease relapse. Inflammatory bowel diseases. 2009; 15(8): 1190-1198. doi: 10.1002/ibd.20933
- Parsi MA, Shen B, Achkar JP, Remzi FF, Goldblum JR, et al. Fecal lactoferrin for diagnosis of symptomatic patients with ileal pouch-anal anastomosis. Gastroenterology. 2004; 126 (5): 1280-1286.
- Walker TR, Land ML, Kartashov A, Saslowsky TM, Lyerly DM, et al. Fecal lactoferrin is a sensitive and specific marker of disease activity in children and young adults with inflammatory bowel disease. Journal of pediatric gastroenterology and nutrition. 2007; 44 (4): 414-422. doi: 10.1097/MPG.0b013e3180308d8e
- Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, et al. Fecal calprotectin more accurately corrects the endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inßammatory bowel diseases. 2013; 19 (2): 332-341. doi: 10.1097/MIB.0b013e3182810066.
- Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, et al. Noninvasive markers in the evaluation of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. The American Journal of Gastroenterology. 2008; 103 (1): 162-169. doi: 10.1111/j.1572-0241.2007.01556.x
- Silberer H, Kuppers B, Mickisch O, Baniewicz W, Drescher M, et al. Fecal leukocyte proteins in the inflammatory bowel disease and irritable bowel syndrome. Clinical laboratory. 2005; 51 (3-4): 117-126.
- Schoepfer AM, Vavricka S, Zahnd-Straumann N, Straumann A, Beglinger C. Monitoring in inflammatory bowel disease activity: Clinical activity is judged to be more relevant than endoscopic severity or biomarkers. Journal of Crohn’s & colitis. 2012; 6 (4): 412-418. doi: 10.1016/j.crohns.2011.09.008
- Burri E, Beglinger C. The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert review of gastroenterology & hepatology. 2013; 8 (2): 197-210. doi: 10.1586/17474124.2014.869476
- Dai J, Liu WZ, Zhao YP, Hu YB, Ge ZZ, et al. Relationship between fecal lactoferrin and inflammatory bowel disease. Scandinavian journal of gastroenterology. 2007; 42 (12): 1440-1444. doi: 10.1080/00365520701427094
- Hayakawa T, Jin CX, Ko SB, Kitagawa M, Ishiguro H. Lactoferrin in gastrointestinal disease. Internal medicine. 2009; 48 (15): 1251-1254. doi: 10.2169/internalmedicine.48.2199


