Research Article
The Hematological parameters of Catfish (Clariasgariepinus) fed Fish Feeds with replaced Premix using Moringa Leaf Meal (MLM)
1Department of Aquaculture and Fisheries Management, University of Ibadan, Ibadan, Nigeria
2Department of Biological Sciences, Bells University of Technology, Ota, Nigeria
*Corresponding author: Omoike Augustine, Department of Biological Sciences, Bells University of Technology, Ota, Nigeria, E-mail: dromoike@yahoo.com
Received: January 15, 2018 Accepted: August 6, 2018 Published: August 11, 2018
Citation: Eyiwunmi FA, Augustine O, Ovie WK. The Hematological parameters of Catfish (Clarias gariepinus) fed Fish Feeds with replaced Premix using Moringa Leaf Meal (MLM). Madridge J Aquac Res Dev. 2018; 2(1): 35-39. doi: 10.18689/mjard-1000107
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
A 56-day growth performance trial was conducted using Moringa oleifera meal (MLM) inclusion as a partial replacement of premix to determine hematological parameters of the African catfish, Clariasgariepinus (juveniles). Four isonitrogenous 40% crude protein rations containing replacement of premix by Moringa Leaf Meal (MLM) treatment I, Control (0% MLM)treatment II (0.5% MLM inclusion), treatment III (1.0% MLM inclusion) and treatment IV (1.5% MLM inclusion) were fed as 5% body weight biomass to triplicate treatment of 30 each of C. gariepinus Juvenile (12.5 ± 0.03g).Treatment IV had the best hematological parameters result, was not significantly different (P>0.05) from other treatments in packed cell volume (PCV) 25.83±1.21; Haemoglobin (Hb): 8.53±0.41 and Red Blood Cell (RBC): 2.79±0.44. Treatment I also had good haemotologicalindices: PCV: 25.17±2.85; Hb: 8.20±0.99; RBC: 2.77±0.13, Platelet: 125.50±8.18. Treatments II and III had higher levels of White Blood Cell (WBC) which ranged from 18.31±1.23 to 18.65±1.83. Hematological parameters results were within the recommended ranges for fish health. This study revealed that moringa leaf meal (MLM) was suitable as replacement forpremix, it enhanced the hematological performance of Clariasgariepinus juveniles. It is therefore recommended at 1.5% inclusion for successful practice of aquaculture due to its vitamin and mineral components.
Keywords: Moringa leaf meal, Hematological parameters Clariasgariepinus, premix.
Introduction
Fish constitutes about 41% of the total animal protein intake by the average Nigerian hence, there is a great demand for fish in the country [1]. With the global population expansion, the demand for high quality food is rising dramatically. Nigeria requires about 2.66million metric tonnes of fish out of which only 0.62million metric tonnes was produced locally [2]. The shortfall in the demand is met through importation, whereby US $594.4million is spent annually on importation of fish and fishery products in Nigeria [3]. Currently, there is significant decrease in fish production from the capture fisheries, while in the culture fisheries, the production level is on the increase with almost 100% increase recorded between 2004 and 2007 [2]. Fish is nutritive, tasty and acceptable with distinctive source of essential mineral, vitamins, fats/oils and amino acid. Aquaculture research has advanced throughout the world and focus has been geared towards increase yield through feed production and feeding practice [4]. Fish nutrition has improved in recent years with the development of balance diets that promote optimal growth and health of fish. Fish production is related the feed supply; feed needed must be nutritionally balanced and formulated to meet the required nutrient of the fish species under culture. The cost of fish feeds mostly determines the viability of aquaculture as it accounts for between 40-75% operation costs of the fish production [3]. There is therefore an urgent need to intensively research into means of redressing feed costs by using local alternative feed ingredients that are common, cheaper and readily available.
The hematological characteristics of fishes are an integral part of evaluating their health status. However the diet composition, metabolic adaptation and variation in fish activity are the main factors responsible for the change in hematological parameters of fish [5]. The use of moringa leaf meal as replacement for premix to checkmating the hematological parameters will be beneficial. Moringaoleifera is one of the worldʼs most useful plants. It is fast growing tree grown in the tropics for human food, livestock forage and medicine dye and water purification.
Therefore, this study was carried out to find out the effect of the partial inclusion of moringa leaf meal as a replacement of premix in fish feed, on the health status of fish using the hematological parameters indications, as a determination of the wellbeing of Clariasgariepinus.
Materials and methods
Experimental Location
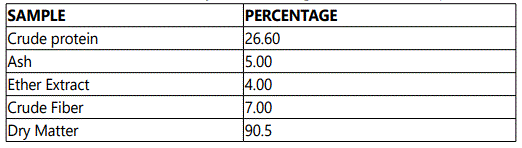
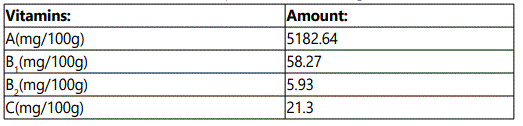
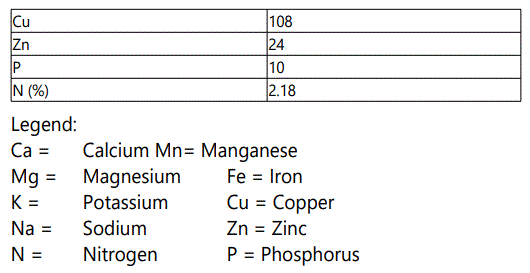
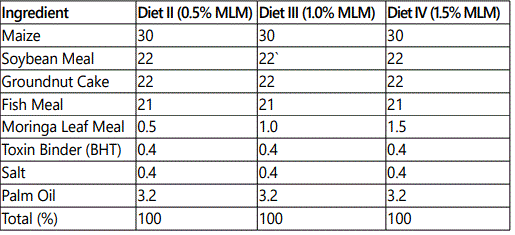
The study was carried out in the Department of Aquaculture and Fisheries, University of Ibadan, Nigeria. The juveniles were procured from a reputable commercial fish hatchery farm within Ibadan. The experimental fishes were first acclimatized to laboratory conditions for two weeks (14days) at a mean temperature of 27.5±0.51o C, and were fed with commercial fish feed (45% C.P) (Coppens). The experimental diet with 40% CP was compounded using the moringa leaf meal inclusion. The analysis of the moringa leaf is stated in Table 1, Table 2 and Table 3. They were later fed twice daily, 7am and 6pm on 5% of the biomass with compounded feeds as shown in Table 4, for fifty six days.

Experimental conditions
The experiments were made up of four (4) treatments.
Treatment I: fed pelleted sinking fish feed (0% Moringa Leaf Meal (MLM) inclusion, as control.
Treatment II: fed 0.5% MLM inclusion, pelleted sinking feed.
Treatment III: fed 1.0% MLM inclusion, pelleted sinking feed
Treatment IV: fed 1.5% MLM inclusion, pelleted sinking feed.
A total of 120 Clariasgariepinus juveniles of a mean initial weight of 12.0± 0.03g were randomly distributed into 12
tanks with 50-L capacity of water. The fish were fed with commercial fish feed (Coppens), during acclimatization and the formulated treatment diets with moringa inclusion and control without moringa leaf meal inclusion for 56 days during the experimental periods.
Processing of Moringa Leaves to Meal (supplement)
Moringa plant was cultivated for the purpose of this research, and was harvested seven months after maturity. The leaves were thoroughly washed with water to remove dirt, drained properly and later shade-dried for two weeks (14 days). Thereafter, the leaves were blended into fine powder and analyzed for proximate composition according to [6]. The parameters of importance were: crude protein, crude fat, crude fibre, moisture content and total ash, vitamins and mineral elements.
Collection and Evaluation of Experimental Fish Blood Samples
Prior to the commencement of the experiment, baseline blood samples were collected from four fish randomly selected from all the fish for hematological analysis, and 2ml of blood was also collected at the end of study (56 days) from the caudal peduncle of fish in each experimental unit [7]. Blood collected were dispensed into tubes (Lithium heparin) containing EDTA an anticoagulant and taken to the haematology unit, veterinary medicine Department, University of Ibadan for determination of packed cell volume (PCV), platelet (PLT) lymphocyte (LYMP) mean cell volume (MCV) haemoglobin (Hb), white blood cells (WBC) and red blood cells (RBC) using the method of [8]. The haematolobin (MCH) and mean cell haemaglobin concentration (MCHC) were calculated using the method described by [9,10].
Haemoglobin Estimation
The haemoglobin concentration estimate was determined using the cyanomethaemoglobin method. 20μl of blood sample was taken from the lithium heparinized tube with the aid of a pipette. Mixing was achieved by slow inversion of the tube, for about 20times with 4.0ml of Drabkinʼs solution. After which the text tube was taken into a calorimeter for reading. The final haemoglobin result was calculated from:

Where:
T = the test absorbance
A = the standard absorbance
C = the concentration of cyanmethaemoglobin
DF = the dilution factor
Red Blood Cell (Erythrocyte)
Commonly available diluents were used for the red blood cell count (RBCC) i.e. a solution of formal-citrate. The blood sample diluted by washing 20μl of blood into a shellback pipette, and into 4.0ml of modified Drabkinʼs fluid to give a final solution of 1 in 20litres. The diluted sample were then mixed and loaded into the haemocytometer. After the cells sedimentation, the number lying on 5 of the 0.04mm2 area were counted, by charging and placing the Neubergerʼs chamber under a microscope and thereafter counted.
The final value was express as the number of the cell per liter.
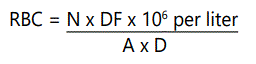
Where:
N = the number of cell counted
DF = the dilution factor
A = the area chamber counted
D = the depth of chamber
White Blood Cell (Leucocytes)
The WBC is determined by drawing the blood sample with the aid of pipette about 20μL and put into test tube and mixed with diluents solution at 0.38ml. This prepared diluents will liase (destroy the red cells) the red blood cells and this make the white cells more readily visible. It was spread on blood film and counted until a minimum of 200 cells have been enumerated.
Packed Cell Volume (PCV)
The PCV (haematocrit) was determined with Wintrobehaematocrit, filled to 10mark, to avoid bubbles according to Wintrobeʼs and Westergreenʼs methods as described by [8], with commercially available heparin capillary tubes of 75mm. this was done by centrifuging the haematocrit at 3000 r.p.pm for 30minutes, further readings were taken. The readings were not the same, centrifugation was repeated until two identical consecutive readings were obtained. This was the time required for pack cell on this particular centrifuge, it was removed from centrifuge and the height of the red cell column noted which is to the button of the buffy layer. The tube was divided into 100 divisions; the height of the column of red cells was read off and was expressed as a fraction of the whole blood.
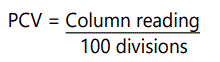
General Observation
Experimental fish in each tank were thoroughly observed daily for any behavioral and morphological changes, injuries and general well-being. All observation and mortalities were recorded. Feed acceptability was low for treatment II, III and IV for the first few weeks, and they later pick-up from day 14 to 56.
Statistical Analysis
Data collected from the experiment were subjected to analysis of variance (ANOVA) test, Duncan Multiple Range Test (DMRT) [11] was used to compare differences among individual meansand the data were analyzed using SPSS 15.0 window version. Differences were considered significant at 0.05 level (P>0.05).
Results and discussion
Proximate Analysis of Experimental Diets
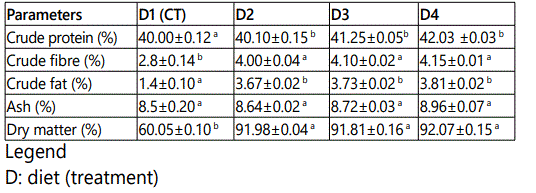
The result of proximate analysis of the experimental diets is shown in table 5 Due to the inclusion of moringa leaf meal (MLM) as premix, in the following proportions 0.5, 1.0 and 1.5 respectively, the crude protein later showed higher values for diet 2, 3 and 4 on pelleted sinking feed after analysis. The (D1: 40%, D2 = 40.25%, D3 = 41.30% and D4 = 42.00%).
Hematological parameters
The hematological result in this study shows the packed cell volume (PCV) values for treatment II was significantly (P>0.05) different but those of treatments III and IV were not significantly different, the values ranges from 23.30±2.98 to 25.83±1.21.
There was no significant difference in the Haemoglobin in treatments I and IV, the values ranged from 8.20±0.99to 8.53±0.41.
Red Blood Cell (RBC) across the treatments (II, III & IV) showed no significant difference (P>0.05), with the highest values obtained in treatment IV (2.79±0.44) and the lowest values in treatment II (2.41±0.68).
White Blood Cell (WBC) were not significantly (P>0.05) different in treatments II and III, Higher values were obtained in treatments II and III, 18.65±1.83 and 18.31±1.23 respectively, and lowest in treatment I, (16.28±0.96).Which was significantly different (P>0.05).
Platelet (PLT) content was not significantly (P > 0.05) different in treatments II, III and IV, compared to treatment I (control). Blood platelets in the control experiment (Treatment I) was higher compared to other treatments.
There was significant (P > 0.05) difference in lymphocyte (LYMP) for treatments I, II and IV, but there was no significant difference in treatments III (P>0.05). The highest values were in treatment III (71.00±3.00). Mean Corpsular Haemoglobin (MCH) of treatments I and IV did not show significant difference (P>0.05).
The other treatments showed significant (P>0.05) difference on MCH and ranges from 3.l7±0.90 (treatment III) and 2.67±0.94 (treatment II). Mean corpsular volume (MCV) was highest in treatment II (100.50±15.24) and lowest in treatment IV (94.00±11.4).There was no significant (P > 0.05) difference in mean Corpuscular Haemoglobin concentration (MCHC) among the various treatments.
Water Quality Analysis
Water quality parameters monitored in this study included temperature, pH and dissolved oxygen. The mean values recorded for each parameter is presented in Table 6
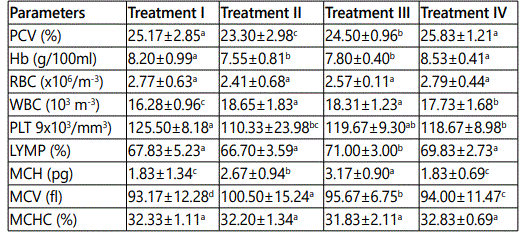
Means with the same superscript along the same row are not significantly different P>0.05)
Legend
PCV = Packed cell volume
Hb = Haemoglobin
RBC = Red Blood Cell
WBC = White Blood Cell
PLT = Platelet
LYMP = Lymphocyte
MCH = Mean corpsular Haemoglobin
MCV = Mean corpsular volumebr
MCHC = Mean corpsular Haemoglobin concentration
Mean temperature value was within the ranges from 26.4±0.37 to 27.7+0.35 throughout the period of the experiment. Mean pH levels varied between the ranges of 7.0±0.02 to 7.6±0.06.Mean dissolved oxygen levels for all reading tanks were measured to be within the ranges 5.7±0.15 to 7.9±0.06.
Proximate Analysis of Experimental Diets
The feeding trials revealed that Clariasgariepinus responded to all the diets, irrespective of their moringa leaf meal inclusion.The hematological parameters of fish are reported to be affected by a range of factors which includes specie, size, age, physiological status, environmental conditions and dietary regime e.g. quality and quantity of food, dietary ingredients, protein sources, vitamins, etc. however, moringa leaf meal inclusion has been used as premix to affect the hematological parameters, which are important and in checkmating the health status of fish. The high crude protein of the pelleted diets was due to the various inclusion levels of moringa leaf meal, which has been shown to reduce amino acid availability [12].The crude fibre, ash, dry matter had higher values in the formulated feeds as compared to the floating feed used for the control experiments. Montagne et al [13] stated that processes involved in floating feed production could increase the soluble fibre content of plant source of feed ingredients thereby reducing their crude fibre content.
Hematological
The hematological indices of blood are useful in monitoring feed toxicity especially with feed constituents that affect the formation of blood. However, all the hematological parameters measured in this study were within the recommended physiological ranges reported for Clariasgariepinus.
The packed cell volume (PCV), Haemoglobin (Hb) and Red blood Cell (RBC) were low in fish fed experimental diets of 0.5% and 1% MLM inclusion (treatment II and III). However, a decrease in the hematological parameters of fishes at significant level (P>0.05) was due to the level of inclusion in the diets. This observation was in support of the works of Osuigwe et al [14], Sotolu and Faturoti [15] who reported the reduction in value of packed cell volume (PCV), Haemoglobin (Hb) and Red Blood Cell (RBC) due to the toxic substance in diet of fish.
There was an increase in White Blood Cell (WBC) and lymphocytes of the experimental fish fed with varying moringa leaf meal, this increment in whole blood cells and lymphocyte in the circulatory system contributed to better survival rate of the experimental fish. This also increased the iron content of the feed which is a major source of haemoglobin (Hb) in fish in the diets constituents with highest level of moringa inclusion. Moringa leaf meal is used as animal feed and most recently consumed as supplement in human diet, and could have led to an increase in the various hematological parameters in this study. However, moringa leaf meal inclusion of 1.5% as premix replacement was the best amongst the treatment.
Conclusion
The study has shown that premix in fish feed could be replaced with Moringaoleifera leaf meal up to 1.5% level in Clariasgariepinus diets without any negative effects on the hematological parameters, health and feed efficiency. The toxicology test has showed that percentage replacement of premix diets would not pose any threat on the blood of fish. The use of moringa leaf meal as vitamin and mineral supplements in fish feed is recommended to strengthen the immunopathological system in fish.
References
- Atanda AN. Fish species diversification in aquaculture for the success of the agriculture transformation agenda. FISON Lecture Series. 2012; 16.
- FDF. Fisheries statistics of Nigeria, Federal Department of Fisheries, Fourth edition. 2008; 32.
- Falaye AE. Fish: A jewel in land and sea environment; An Inuagural lecture, Ibadan University Press. 2013; 7-31.
- KangʼOmbe J, Likongwe JS, Eda H,Mtimuni JP. Effects of varying dietary energy level on feed intake, feed conversion, whole body composition and growth of Malawian tilapia, Oreochromisshiranus Boulenger. Aqua Culture Research. 2007; 38(4): 373-380. doi: 10.1111/j.1365-2109.2007.01676.x
- Keri-Alhadi I, Aziz Bin A, Abol-Munati AB. Haematological changes in Nile Tilapia (Oreochromisniloticus) fed with varying dietary maltose levels. World Journal of Fish and Marine Sci. 2012: 4(4): 376-381.
- Association of Official Analytical Chemists 17thed, AOAC Wasshington DC. 2000; 21-447.
- Joshi PK, Bose M, Harish D. Changes in certain haematological parameters in siluroid catfish Clariasbatrachus (Linn) exposed to cadmium chloride. Pollution Resources. 2002; 21(2): 129-131.
- Blaxhall PC, Daisley KW. Routine haematological methods for use with fish blood J. fish Boil. 1973; 5(1): 771-781. doi: 10.1111/j.1095-8649.1973. tb04510.x
- Androw BL. Experimental physiology 9th ed. 1972; 1228.
- Merghami TH. The core of medical physiology 3rd ed. 2010; 156-158.
- Duncan DB. Multiple range and multiple F tests. Biometrics. 1955; 119(1): 1-42.
- Singh S, Wakeling L,Ganalath S. Retention of essential amino acids during extrusion of protein and reducing sugars. J Agric Food Chem. 2007; 55(21): 8779-8786. doi: 10.1021/jf071769z
- Montagne L, Pluke JR, Hampson DJ. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003; 108(1): 95-117. doi: 10.1016/S0377-8401(03)00163-9
- Osuigwe DI, Nwosu C, Ogunji JO. Preliminary Observation on some heamatological parameters of juvenile Heterobranchuslongfilis fed different dietary levels of raw and boiled jack bean (Canavaliaensiformis) seed meal. Conference on International Agricultural Research for Development. 2005; 24(2): 19-26. doi: 10.4314/nvj.v24i2.3450
- Sotolu AO, Faturoti EO. Growth performance and hematological effects of varying dietary processed Leucaena leucocephala seed meal in Clariasgariepinus juveniles. African J Food, Agriculture, Nutrition and Development. 2011; 11(1): 4547-4557.


