Research Article
Stability Indicating New-UPLC Method for Determination of Dimethyl Fumarate in their Pure and Capsule Dosage Form
HIKMA Pharmaceutical Company, Beni-Suef, Egypt
*Corresponding author: Mahmoud A Mohamed, HIKMA Pharmaceutical Company, Beni-Suef, Egypt, Tel: +2 01124767625, Email: ch.mahmoud88@gmail.com & mmabdelfatah@hikma.com
Received: May 20, 2018 Accepted: September 18, 2018 Published: September 22, 2018
Citation: Mohamed MA. Stability Indicating New-UPLC Method for Determination of Dimethyl Fumarate in their Pure and Capsule Dosage Form. Madridge J Anal Sci Instrum. 2018; 4(1): 82-87. doi: 10.18689/mjai-1000116
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: The current study aimed to develop and validate New-UPLC assay and dissolution methods for determination of Dimethyl Fumarate in their capsule dosage form.
Method: Chromatographic system was performed on the Waters Acquity BEH-C8 (50 mm× 2.1 mm) 1.7µm using isocratic systems Water : Acetonitrile: Phosphoric acid 85% (70:30:1 v/v) at flow rate of 0.5 mL/min, injection volume 0.8 µL, UV detection at 210 nm, Column Oven Temperature25 ºC and Autosampler Temperature 15 ºC.
Results: This method was validated according to ICH requirements for new methods, which include accuracy, precision, selectivity, robustness, ruggedness, LOD, LOQ, linearity and range. Linear relationships were obtained in the ranges of 5-160 µg/mL with correlation coefficients of 0.9997. The forced degradation studies as acidity, alkalinity, oxidation, heat, and thermal, humidity and photo degradation were performed according to ICH guidelines.
Conclusion: New, simple, accurate, economical and stability-indicating RP-UPLC method was developed and validated for estimation of Dimethyl Fumarate in their capsule dosage form.
Keywords: Dimethyl Fumarate; assay; RP-UPLC; Stability indicating method; Capsule dosage form.
Introduction
Dimethyl Fumarate (DMF) represents the third oral agent was approved by the United States (US) Food and Drug Administration (FDA) on March 27, 2013, for the treatment of patients with relapsing forms of Multiple Sclerosis (MS).The mechanism by which DMF exerts its therapeutic effect in multiple sclerosis is unknown. DMF and the metabolite, monomethyl fumarate (MMF), have been shown to activate the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway in vitro and in vivo in animals and humans. The Nrf2 pathway is involved in the cellular response to oxidative stress. MMF has been identified as a nicotinic acid receptor agonist in vitro. TECFIDERA contains DMF (Figure 1)
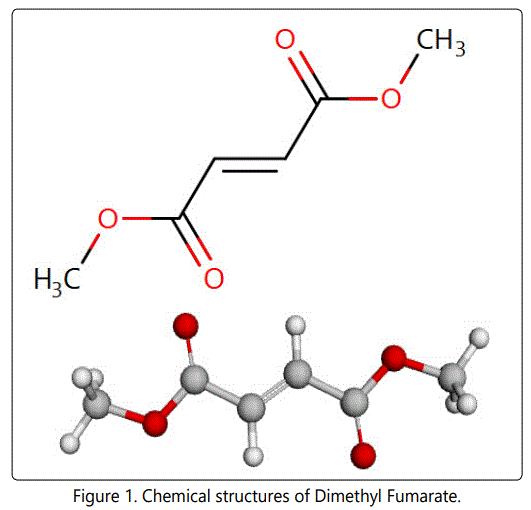
Which is also known by its chemical name, dimethyl (E) butenedioate. Its empirical formula is (C6H8O4), DMF is a white to off-white powder that is highly soluble in water with a molecular mass of 144.13. TECFIDERA is provided as hard gelatin delayed-release capsules for oral administration, containing 120 mg or 240 mg of dimethyl fumarate consisting of the following inactive ingredients: microcrystalline cellulose, silicified microcrystalline cellulose, croscarmellose sodium, talc, silica colloidal silicon dioxide, magnesium stearate, triethyl citrate, methacrylic acid copolymer -Type A, methacrylic acid copolymer dispersion, simethicone (30% emulsion), sodium lauryl sulphate, and polysorbate 80. The capsule shell, printed with black ink, contains the following inactive ingredients: gelatin, titanium dioxide, FD&C blue 1; brilliant blue FCF, yellow iron oxide and black iron oxide [1]. DMF is non-official in BP, EP [2-3] and United States Pharmacopeia (USP) [4]. Literature review showed that few analytical methods have been described for the estimation of DMF including spectrophotometric [5-6], gas chromatography (GC) [7-11], high performance liquid chromatography (HPLC) [12-17], electrochemical and voltammetric methods [18] have been reported for the estimation of DMF in pure or in dosage forms.
To the best of my knowledge there is no RP-UPLC Method was reported for estimation of DMF in their pure and capsule dosage forms. The present work aims to develop a simple, sensitive, short retention time and accurate RP-UPLC method for the estimation of Dimethyl Fumarate in their pure and capsule dosage forms with high sensitivity, selectivity that are required to be in routine quality control analysis, forced degradation studies and validate the developed methods according to ICH guidelines [19].
Experimental Chemicals and Reagents
Pure Samples
Pure sample of Dimethyl Fumarate was kindly supplied by Hikma Pharmaceutical Company, with claimed purity of 99.6%. According to manufacturer certificates of analysis.
Pharmaceutical Dosage Form
Dimethyl Fumarate ® 120mg and Dimethyl Fumarate ® 240 mg CAP were manufactured by hikma Pharmaceutical Company. Each one tablet is claimed to contain 120 & 240 mg of Dimethyl Fumarate.
Chemicals
Acetonitrile, Methanol HPLC-grade, Water (Ultrapure) and Ortho Phosphoric Acid 85% (Analytical grade) were procured from (scharlau, Spain).
Instrumentation
i. The Waters® ACQUITY UPLC® System provides an integrated configuration for solvent and XYZZ sample management designed for use with ACQUITY UPLC chemistries. The core ACQUITY UPLC System comprises a Binary Solvent Manager, a Sample Manager with integral Column Heater, and a Solvents Tray with Empower™ 3 Software. Separation and quantitation was performed on a C18 column (50 mm * 4.6 mm i.d, 1.7 µm particle size) (USA).
ii. The UV- 1800 double beam UV-Visible spectrophotometer (Shimadzu-Japan) with highest resolution which spectral bandwidth is (1 nm from 190- 1100 nm range) was used for all absorbance measurements. Matched with 1cm quartz cells. Perform data analysis by software (UV-Probe 2.5.2).
iii. pH meter METTLER TOLEDO Seven Compact.
Mobile Phase Preparation
Mix 700 mL of water with 300 mL of acetonitrile, add 1.0 mL Ortho Phosphoric acid 85%. Filter through 0.2 µm membrane filter and degas.
Diluent: Methanol HPLC grade.
HPLC Chromatographic Conditions
Chromatographic separation was performed on column Water C18 (50 X 4.6 mm i.d, 1.7 µm particle size) (USA).Using a mobile phase mixture of 700 mL of water with 300 mL of acetonitrile, add 1.0 mL Ortho Phosphoric acid 85% at ambient temperature, at flow rate of 0.5 mL/min, injection volume 0.8 µL, UV detection at 210 nm, Column Oven Temperature 25 ºC and Autosampler Temperature 15 ºC.
Preparation of Standard Solution
Preparation of Standard Stock Solution: (Conc. of DMF is
1000 μg /mL)
Weigh Accurately 100 mg of DMF in to 100 mL volumetric
flasks, add 70 mL of diluent and sonicate to dissolve. Make up
to the mark with diluent and mix.
Working Standard Solution of DMF: (Conc. of DMF is 120 μg
/mL)
Transfer Accurately 24 mL of standard stock solution in to
200 mL volumetric flasks, add 150 mL of diluent and sonicate
to dissolve. Make up to the mark with diluent and mix. The
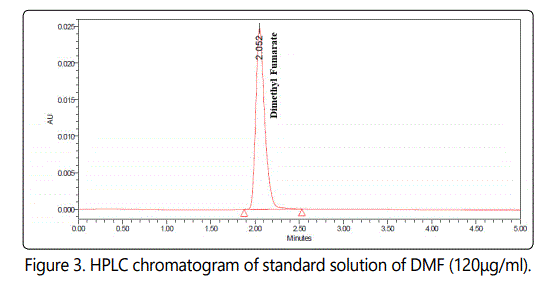
chromatogram obtained was shown in (Figure 3).
Construction of Calibration Curves
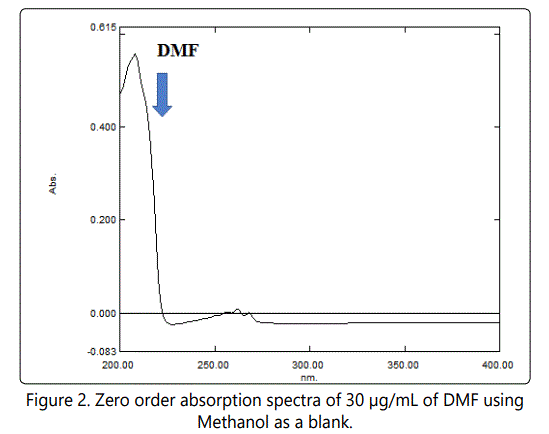
Different concentration of DMF equivalent to 5–160, were separately weighted from their respective stock standard into separate series of 100 mL volumetric flasks, and the volumes were made up to volume with diluent. Duplicate 0.8 µL injections were made for each concentration maintaining the flow rate at 0.5 mL/ min and the effluent was UV- scanned at 210 nm (Figure 2).
The chromatographic separation was performed following the procedure under chromatographic conditions. The chromatograms were recorded and the peak areas of DMF were determined and the calibration curves relating the obtained integrated peak area to the corresponding concentrations were constructed and the regression equations were performed.
Application to Pharmaceutical Formulation
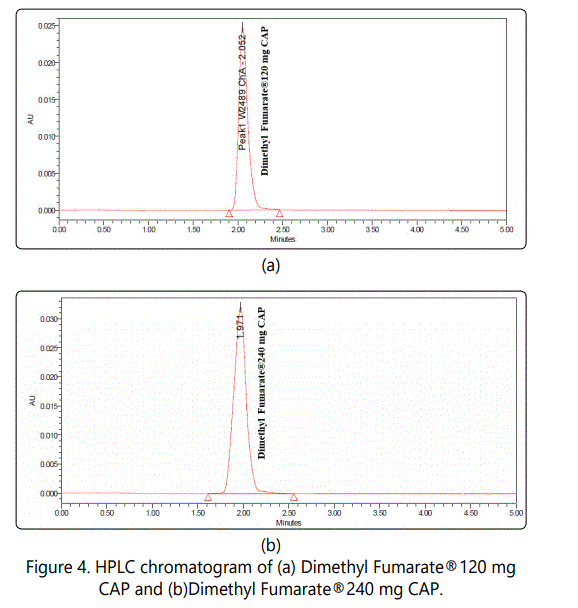
Weight 20 Capsules and calculate the average content per capsule. Weight accurately about the equivalent to 240 mg of Dimethyl Fumarate and transfer into a 100 mL volumetric flask with the aid of 75 mL methanol. Sonicate for about 30 minutes with hand shaking every 5 minutes. Complete up to volume using methanol and mix well. Transfer 2.5 mL from resulting solution into a 50 mL volumetric flask. Complete up to volume using methanol and mix well. The chromatogram obtained was shown in (Figure 4 a, b).
Standard addition technique has been carried out to assess validity of the method by spiking the pharmaceutical formulation with known amount of standard solution of DMF. The recovery of the added standards was then calculated after applying the proposed methods.
Results
The chromatogram obtained at retention time 2.011±0.033 min for DMF and No interfering peak occurred.
Discussion
Methods Development and Optimization
Different developing systems of different compositions and ratios were tried including: methanol: water (50:50, v/v), Acetonitrile: water (50:50, v/v). Methanol: ACN (30:70, v/v) gave poor peak shape, Water: Acetonitrile: Phosphoric acid 85% (70:30:1 v/v). It was found that presence of Phosphoric acid 85% in the developing system is essential for improving tailing of peaks and good system for elution of drug with good peak shape as well as retention time. Different flow rates were tried, scanned wavelengths (210,220, 254, and 265 nm) were also tried. Preliminary studies involved trying C18 reversed-phase columns. The best developing system was Water: Acetonitrile: Phosphoric acid 85% (70:30:1 v/v) at flow rate 0.5 mL/min and at wavelength 210 nm using column Waters Acquity BEH-C8 (50 mm × 2.1 mm) 1.7µm, Column Oven Temperature 25 ºC and Autosampler Temperature 15 ºC.
This selected developing system allows good separation between the drug and its degradation with good Rt values without tailing of the separated bands and good theoretical plates.
Method Validation
The method was validated, in accordance with ICH guidelines (ICH Q2R1), for system suitability, precision, accuracy, linearity, specificity, ruggedness, robustness, LOD and LOQ [19].
Linearity and Range
The linearity of the proposed methods was obtained in the concentration range (5-160 μg/mL) for DMF. Calibration graphs were plotted on the basis of analysis of each calibration solution. The coefficient of regression obtained was 0.9997 for DMF. The slope obtained was 685.5703 and intercept 147.4020. Linearity results were shown in Table1.
Repeatability
Repeatability of the method was evaluated by calculating the RSD of the peak areas of six replicate injections for the standard concentration (100%) of DMF. Results were examined as % RSD values of concentration of drugs determined. Low values of % RSD <2 indicate high precision of the method as shown in Table 1.
Detection and Quantitation Limits
These approaches are based on the Standard Deviation of the Response and the Slope. A specific calibration curve should be studied using samples, containing an analyte in the range of LOD and LOQ. The residual standard deviation of a regression line or the standard deviation of y-intercepts of regression lines may be used as the standard deviation. LOD=3.3×σ /slope and LOQ =10×σ /slope, where σ = the standard deviation of the response Table 1.
Accuracy and Recovery
Accuracy of the proposed methods was calculated as the percentage recoveries of pure samples of the studied drugs. Accuracy is assessed using three different concentrations covering the specified range from (5-160 μg/mL) (i.e. three concentrations and three replicates). Concentrations were calculated from the corresponding regression equations. The mean % recoveries for DMF were between 98.0% to 102% and were shown in Table 2.
Accuracy was further assessed by applying the standard addition technique to pharmaceutical dosage form, where good recoveries were obtained revealing that there was no interference from excipients, Table 3.
Formulation Assay
The validated method was applied to the determination of DMF in commercially available Dimethyl Fumarate® 120&240 mg CAP. The result of the assay undertaken yielded 101.545 and 103.61% of the label claim for Dimethyl Fumarate® 120&240 mg CAP, respectively. The results of the assay indicate that the method is selective for the analysis of Dimethyl Fumarate® 120&240 mg CAP without interference from the excipients used to formulate and produce these tablets. The results were displayed in Table 4.
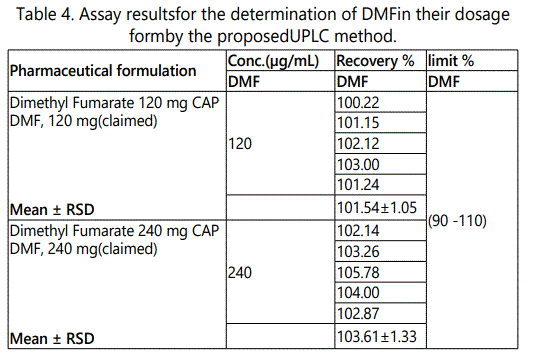
Intermediate Precision (Ruggedness)
Intermediate precision expresses within-laboratories variations: different days, different analysts, different equipment’s, etc. Good results were obtained and presented in Table 5.
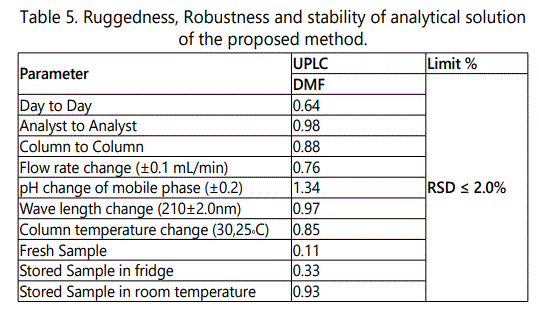
Robustness
The robustness of the proposed methods was evaluated in the development phase where the effects of different factors on method were studied to obtain the optimum parameters for complete separation. Robustness of the method was studied by deliberately varying parameters like flow rate (±0.1 mL/min) and studying the effect of changing mobile phase pH by (± 0.2), acetonitrile composition (±5%) and column temperature changed (±5°c). The low values of the %RSD, as given in Table 5, indicated the robustness of the proposed methods.
Stability of Analytical Solution
To demonstrate the stability of standard solution during analysis, solution was analyzed over a period of 24 hr at room temperature and refrigerator. The results showed that for all the solutions, the retention times and peak areas of DMF remained almost unchanged (RSD<2.0%) indicating that no significant degradation occurred within this period, i.e. both solutions were stable for at least 24 h, which was sufficient to complete the whole analytical process. The results were displayed in Table 5.
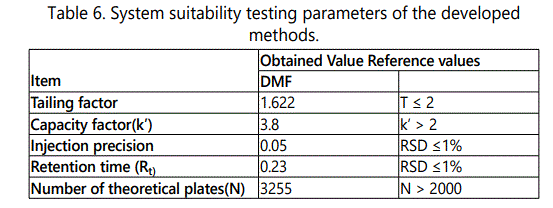
System Suitability
System suitability testing is an integral part of many analytical procedures. The tests are based on the concept that the equipment, electronics, analytical operations and samples to be analyzed constitute an integral system that can be evaluated as such. System suitability was checked by calculating tailing factor (T), column efficiency (N), resolution (Rs) factors. All calculated parameters were within the acceptable limits indicating good selectivity of the methods and ensuring system performance, Table 6.
Specificity
Placebo Interference: Specificity was tested against standard compounds and against potential interferences in the presence of placebo. No interference was detected at the retention time of DMF in placebo solution.
Forced Degradation: The Forced degradation of API was carried out as per ICH guidelines (ICH, Q2B) in acid, base, water, oxidation, photo, heat and thermal. The results were displayed in Table 7.
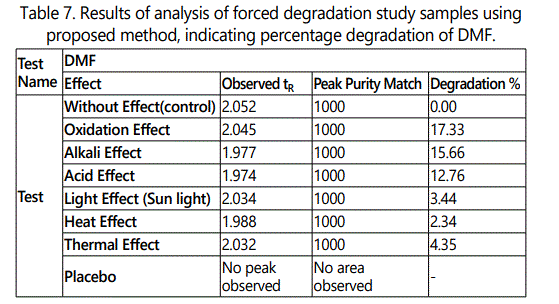
Acid Degradation
Weigh accurately 24 mg of DMF into 200 mL volumetric flask, dissolved in 150 mL of diluent and 5 mL of 0.1N aqueous HCl solution and closed the volumetric flask by stopper. Heated the solution at 85°C in water bath with stirring up to 24hr. neutralized with 0.1N aqueous NaOH solution and made up to the mark with diluent, and injected into the chromatographic system, and calculated the percent of degradation.
Base Degradation
Weigh accurately 24 mg of DMF into 200 mL volumetric flask, dissolved in 150 mL of diluent and 5 mL of 0.1N aqueous NaOH solution and closed the volumetric flask by stopper. Heated the solution at 85°C in water bath with stirring up to 24 hr. neutralized with 0.1N aqueous HCl solution and made up to the mark with diluent, and injected into the chromatographic system, and calculated the percent of degradation.
Peroxide Degradation
Weigh accurately 24 mg of DMF into 200 mL volumetric flask, dissolved in 150 mL of diluent and 3 mL of 3.0% aqueous H2O2 solution and closed the volumetric flask by stopper. Kept the solution at room temperature up to 48 hr. injected into the chromatographic system and calculated the percent of degradation.
Water Degradation
Weigh accurately 24 mg of DMF into 200 mL volumetric flask, dissolved in 150 mL of diluent and 30 mL of purified water and closed the volumetric flask by stopper. Heated the solution at 50-60°C in water bath with stirring up to 48 hr. injected into the chromatographic system and calculated the percent of degradation.
Photo Degradation
Photo degradation is carried out by keeping the powder of DMF under sun light for 48 hours then accurately transfers 24 mg of DMF into 200 mL volumetric flask, dissolved in 150 mL of diluent and diluted up to the mark with the same solvent. Injected into the chromatographic system and calculated the percent of degradation.
Heat Degradation
Heat stress studies were carried out by keeping the powder of DMF in drying oven at 80ºC for 8 hr. then accurately transfers 24 mg of DMF into 200 mL volumetric flask, dissolved in 150 mL of diluent and diluted up to the mark with the same solvent. Injected into the chromatographic system and calculated the percent of degradation.
Thermal Degradation
Thermal stress studies were carried out by keeping the powder of DMF in climatic chamber at storage condition 40ºC ± 2 &75% ± 5% RH for one month then accurately transfers 24 mg of DMF into 200 mL volumetric flask, dissolved in 150 mL of diluent and diluted up to the mark with the same solvent. Injected into the chromatographic system and calculated the percent of degradation.
Conclusion
In present work RP-UPLC method was developed for estimation of Dimethyl Fumarate in pure and capsule dosage form. This method is very simple, precise, specific, highly accurate and less time consuming for analysis, low cost and rapid. The results of stress testing that have been undertaken according to the International Conference on Harmonization (ICH) guidelines revealed that Dimethyl Fumarate was found to be stable under heat, photo and thermal condition, and labile under acid, base, water and oxidation condition. Based on the above results, the analytical method is valid, fit for use and can be used for regular routine analysis and stability study.
Acknowledgment
First and foremost, I thank Allah the most Gracious and the most Merciful. A deep appreciation goes to my wife for her cooperation, continuous advice and real assistance she kindly offered through all stages of the work.
Conflict of Interest
The author declares no conflict of interest.
References
- Description and Clinical Pharmacology for dimethyl fumarate Drug U. S. Food and Drug Administration. 2013.
- British Pharmacopoeia, Stationary Office, Medicines and Healthcare Products Regulatory Agency, London. 2017.
- European Pharmacopeia, 2014. 8th edition.
- United States Pharmacopoeia 41 Revision, NF 36, 2018; the United States Pharmacopoeia Convention Inc.
- Zhang L, Yu BJ, Du CH. Determination of Dimethyl Fumarate Purity by UV Spectrophotometry. Petrochemical Technology. 1999; 28: 477-479.
- Baojie Y, Long Z, Changhai D. Determination of the purity of dimethyl fumarate by UV spectrophotometry. Guangzhou Chemistry. 1999; 1: 007.
- Lamas JP, Sanchez-Prado L, Regueiro J, Llompart M, Garcia-Jares C. Determination of dimethyl fumarate and other potential allergens in desiccant and antimould sachets. Analytical and bioanalytical chemistry. 2009; 394(8): 2231-2239. doi: 10.1007/s00216-009-2923-5
- Stefanelli P, Barbini DA, Girolimetti S, Santilio A, Dommarco R. Determination of dimethyl fumarate (DMFu) in silica gel pouches using gas chromatography coupled ion trap mass spectrometry. Journal of Environmental Science and Health Part B. 2011; 46(4): 341-349. doi: 10.1080/03601234.2011.559893
- Zhao Y, Qi X. Determination of dimethyl fumarate in leather and textiles by gas chromatography-tandem mass spectrometry with solid phase extraction. Se Pu. Chinese journal of chromatography. 2010; 28(1): 54-58.
- Kawakami T, Isama K, Matsuoka A, Nishimura T. Determination of dimethyl fumarate and other fumaric and maleic acid diesters in desiccants and consumer products in Japan. Journal of Health Science. 2011; 57(3): 236-244. doi: 10.1248/jhs.57.236
- Narizzano R, Risso F, Venturelli G, Devia C, Carlini E, Maggiolo S. Gas-chromatography–mass spectrometry analysis of dimethyl fumarate in consumer products. Journal of Chromatography A. 2009; 1216(39): 6762- 6766. doi: 10.1016/j.chroma.2009.07.072
- Adnan MA, Javali C. Development and Validation of Analytical Method for Simultaneous Estimation of Dimethyl Fumarate and Ondansetron. World Journal of Pharmacy and Pharmaceutical Sciences. 2017; 3: 4. doi: 10.20959/ wjpps20177-9470
- Nagaraju P. Development and Validation of RP-HPLC Method for Estimation of Quetiapine Fumarate in Pharmaceutical Formulations. Pharmaceutical Methods. 2015; 6(2): 105-108.
- Hemant KJ, Archana AG. Stability Indicating Rp-Hplc Assay Method for Estimation of Dimethyl Fumarate in Bulk and Capsules. Int J App Pharm. 2017; 9(5): 121-127. doi: 10.22159/ijap.2017v9i5.20944
- Adnan MA, Javali C. Development and validation of analytical method for simultaneous estimation of dimethyl fumarate and dimenhydrinate in tablet dosage form. World Journal of Pharmacy and Pharmaceutical Sciences. 2017; 6(10): 585-594. doi: 10.20959/wjpps201710-9679
- Gennari O, Montesano D, Salzano A, Albrizio S, Grumetto L. Determination of dimethyl fumarate in desiccant and antimould sachets by reversedphase liquid chromatography. Biomedical Chromatography. 2011; 25(12): 1315-1318. doi: 10.1002/bmc.1602
- Suneetha A, Raja RK. Comparison of LC-UV and LC–MS methods for simultaneous determination of teriflunomide, dimethyl fumarate and fampridine in human plasma: application to rat pharmacokinetic study. Biomedical Chromatography. 2016; 30(9): 1371-1377. doi: 10.1002/bmc.3694
- Puglisi VJ, Bard AJ. Electro hydrodimerization Reactions II. Rotating Ring-Disk Electrode, Voltammetric and Coulometric Studies of Dimethyl Fumarate, Cinnamonitrile, and Fumaronitrile. Journal of the Electrochemical Society. 1972; 119(7): 829-833.
- ICH, Q2 (R1) Validation of Analytical Procedures: Text and Methodology. ICH Harmonized Tripartite Guideline, 2005.


