Research Article
Higher quaternary ammonium salts with the sterically accessible exchange center: application for development of Selenate selective electrodes
Belarusian State University, Republic of Belarus, Russia
*Corresponding author: Yu V Matveichuk, Belarusian State University, Leningradskaya str, 14, Minsk, Republic of Belarus, 220030, Russia, Email: Yu_Matveychuk@mail.ru;
Received: October 31, 2017 Accepted: November 17, 2017 Published: November 23, 2017
Citation: Matveichuk YV, Rakhman′ko EM. Higher quaternary ammonium salts with the sterically accessible exchange center: application for development of Selenate selective electrodes. Madridge J Anal Sci Instrum. 2017; 2(1): 35-40. doi: 10.18689/mjai-1000108
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
A new film selenate selective electrode has been developed, with the following
membrane composition: (3,4,5-tris-dodecyloxybenzyl)tetraoxyethyl trimethyl ammonium
chloride (5% w/w) as an electroactive component, (poly)vinyl chlorid (33% w/w) as a
membrane matrix, 1-bromonaphtalene (42% w/w) as a plasticizer, (4-trifluoroacetyl)
benzoic acid heptyl ester (20% w/w) as a neutral anion carrier. The electrode
characteristics are as follows: linear range - 2·10-6-10-2 M by Na2SeO4; line plot slope
26,0±0,3 mV/decade; lower detection limit 1·10-6 M by Na2SeO4; working pH range 2,15
- 6,3; potential drift 0,9 mV/h; response time 15 - 90 s; selectivity in presence of chloride,
bromide, sulfate and other ions; lifetime up to 3 weeks. The electrode developed has
been tested on model solutions using the standard addition method in the calibration
plot variant, and bracketing technique.
Keywords: Selenate Selective Electrode; Sterically Accessible Quaternary Ammonium
Salts; (4-trifluoroacetyl) Benzoic Acid Heptyl Ester.
Introduction
Selenium is a necessary trace element for plants, animals and humans. It occurs in
nature mainly as selenides, selenites and selenates, and sometimes in the elementary
state. The majority of selenides and selenium element itself have low toxicity due to their
limited bioaccessibility, while selenites and selenates are toxic and act similarly to arsenic
compounds, according to [1,2]. Selenium concentration and form of its occurrence in
waters of various geochemical origin depends upon their mineralization, redox potential,
pH, iron content, proximity of oilfields etc.
For selenium determination, the following methods are currently in use: atomic
adsorption spectroscopy, partition and ionic chromatography, stripping voltammetry,
kinetic, fluorescent, photometric analysis, gas chromatography-mass spectrometry etc,
as described in [1-7]. Despite the high sensitivity (approximately 10-6-10-5 mg/L), these
methods often prove inefficient for direct selenium determination in complex objects.
With background content of the analyte, the relative error can be as much as 50%.
Moreover, the above methods require complicated sample preparation, use of expensive
equipment, specialized reagents and, sometimes, chemical pre-treatment such as
reduction of Se (VI) into Se (IV). For instance, in Reference1 it is reported that, in mineral
waters of Caucasian region, selenium (IV) and selenium (VI) are present in comparable
amounts. To convert Se (VI) into Se (IV), authors propose reduction with concentrated
hydrochloric acid under heating, followed by Se (IV) determination by extraction redox
photometry.
In this respect, ionometry, with its obvious objective
advantages such as simple and quick sample preparation,
wide range of concentrations determined and low equipment
cost, looks very promising. An additional benefit is that both
membranes and ion selective electrodes (ISE) themselves are
easy to make and can be produced in any laboratory.
ISE developments for selenium determination are few and
limited only to selenite ions, as mentioned in [8-10]. No
information on selenate selective electrodes, either coated
wire, film or solid contact type, or references thereof, has been
found in the literature [11-18]. On the other hand, determination
of selenium in selenate form is preferable in terms of greater
stability of Na2SeO4 solutions compared to Na2SeO3. Long
chain quaternary ammonium salts (QAS) proved themselves as
promising materials for making anion selective ISEs. It has been
believed that they uniformly have poor extraction capacity
toward hydrophilic double charged anions (sulfate, selenate,
carbonate etc.) due to strong hydration of these ions and steric
hindrance by long hydrocarbon chains preventing simultaneous
approach of two QAS cations to the double charged anion. The
above is true, however, only for QAS cations having all four
long-chain (no shorter than C3) hydrocarbon substituents at
the nitrogen atom. Therefore, it is interesting to test some new
QAS, with improved steric access to the exchange center,
against double charged anions.
It should be noted that the most spectacular successes in
developing the ion-selective electrodes with nonstandard
(anti-Hofmeister) selectivity were achieved with the aid of
neutral anion carriers such as (4-trifluoroacetyl) benzoic acid
heptyl ester (TFABAHE). That is why this compound was
deemed necessary to use in the membrane of selenate
selective electrodes.
Therefore, the goal of this work is to develop the new
selenate selective electrode of high analytical performance
based on long-chain QAS and TFABAHE. Several recently
synthesized (as described in [19, 20]) long-chain QAS with
high steric accessibility of the exchange center such as
(3,4,5-tris-dodecyloxybenzyl(oxyethyl)n trimethyl ammonium chloride
((oxyethyl)nTM), where n = 2-4); and 4-(3,4-bis-hexadecyloxyphenyl)
butyl trimethyl ammonium bromide (BHPBTM) have been
tested as anion exchangers for the first time.
Experimental
(Oxyethyl)nTM; BHPBTM; trinonyl octadecyl ammonium
iodide (TNODA), 3,4,5-tris-dodecyloxybenzyl tributyl
ammonium chloride (TB), 3,4,5-tris-dodecyloxybenzyl triethyl
ammonium chloride (TE) and 3,4,5-tris-dodecyloxybenzyl
trimethyl ammonium chloride (TM) were used as electroactive
membrane components; dibutyl phtalate (DBP, Sigma-Aldrich),
1-bromonaphtalene (1-BN) p.a., o-nitrophenyl decyl ether
(o-NPDE), bis(2-ethylhexyl) decanedioate (BEHD), didecyl
phthalate (DDP) as plasticizers; TFABAHE as the neutral anion
carrier. Synthetic procedures for quaternary ammonium salts
have been presented on Figure 1 and described in full detail
in [19, 20]. Structures of all new compounds have been
confirmed by elemental analysis, NMR and IR spectra, which are also given in the same [19, 20]. Solutions for ionometry
were prepared from K2SeO4 p., K2SO4 p., Н3РО4 p., KCl p.a.,
NaBr p. and KNO3 p.a.
ISE membranes contained the following components:
(poly)vinyl chloride (PVC) 33% w/w, the ion exchanger (QAS)
5% w/w, the neutral anion carrier (TFABAHE) 20% w/w; and
the plasticizer - 42%, with tetrahydrofurane (Fluka AG) as a
solvent. They have been made according to a standard
procedure described in [21]. All components were weighed
precisely, dissolved in THF and mixed together. The obtained
mixture was poured into the glass ring fixed on the glass plate
and the solvent was allowed to evaporate overnight.
Membrane disks (approximately 0.5-0.6 mm thick) were cut
out from the master membrane and glued on the top of the
PVC tubes with PVC-THF composition. and for the membrane
composition.
The above proportions of membrane components have
been selected on the basis of our previous works. In particular,
in the [22] it has been shown that, for the membrane to be
mechanically strong enough, the PVC content in the
membrane should be no less than 33% w/w. From preliminary
experiments it has been established that the most stable
performance of the ISE (potential reproducibility) is achieved
at 5% w/w of QAS. The effect of the neutral anion carrier
content on the analytical characteristics of ISE reversible to
doubly charged inorganic ions, including selenate ions, was
studied in Reference23, and it has been demonstrated that the
optimum content of the neutral carrier is 20% w/w. The rest of
the membrane, i.e. 42% w/w, is the plasticizer.
All newly made membranes have been soaked for 1-2
days in 1·10-1 M solutions of potassium selenate. The mixture
of potassium selenate (1·10-2 M) and potassium chloride
(1·10-3 M) was used as an internal solution for all ISEs. In
selenate solutions, pH was kept about 3.2±0.1 using
phosphoric acid solution, in order to remove the interference
from carbonates and for better reproducibility. The same pH
value was kept also in the interfering ion solutions while
studying the selectivities of ISEs developed. ISE calibration
was done by the double dilution method.
Activities of SeO42- ions in calibration solutions were
calculated according to the Debye-Hückel theory (for 20°C),
as described in Reference24. Potentiometric selectivity
coefficients were determined by the separate solutions
method in the equal potential variant according to the
formula given in [25].
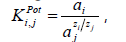
where аi is the activity in the main ion solution at the Е
potential, M; аj is the activity in the interfering ion solution at
the Е potential, M; zi and zj are charges of the main and the
interfering ions, respectively.
Sulfate, chloride, nitrate and bromide ions were selected
as the interference, being the most frequently found in real
objects. Slopes in the interfering ion solutions were,
respectively, 24-25.5 mV/decade for sulfate, 50-51 mV/decade for chloride, 51-52 mV/decade for bromide and 49-50
mV/decade for nitrate. Other characteristics were determined
as recommended in [21].
The potential of the electrochemical cell was measured by the I-160.1MP (Republic of Belarus, Gomel Plant of Measuring Devices) ionometer at 20±1°С. The EVL-1M3.1 silver chloride electrode (Republic of Belarus, Gomel Plant of Measuring Devices) was used as a reference, and the ESL-43-07SR glass electrode (Republic of Belarus, Gomel Plant of Measuring Devices) - for pH measurement.
Results and Discussion
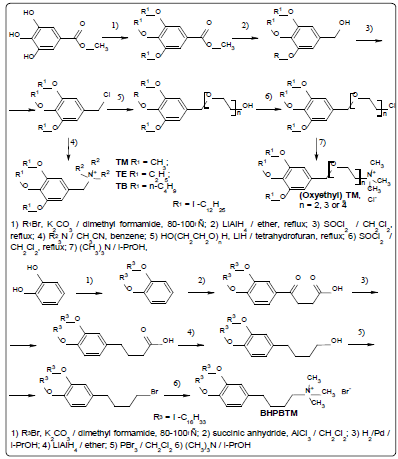
Selecting the working pH range. Figure 2 shows that the
potential of the SeO42- selective electrode (based on
(oxyethyl)2TM, TFABAHE and 1-BN) is stable in the pH range
from 2.25 to 6.3 and equals -217±0.4 mV (at C(K2SeO4)=2.5·10-3
M).
The ISE potential drops at pH>6.3, due to interference of
hydrogen carbonate and hydroxide ions. At pH below 2.25
selenate ions gradually convert to hydrogen selenate ions
(since lgКa(НSeO4−)= -1.8, according to Reference24, about
20% of Se (VI) exist as НSeO4− at рН=2.25).
As it was noted above, the pH value for potentiometric
measurements has been maintained at 3.2±0.1, excluding
interference from hydrogen carbonate, sulfite, selenite,
sulfide, hydrogen phosphate, fluoride etc. anions, as well as
the majority of organic anions such as acetate, oxalate etc.,
due to their protonation. This makes unnecessary taking their
interference into account, as it has been done in [8-10].
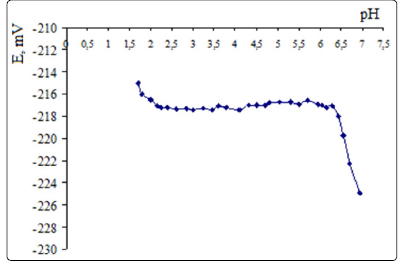
Lower detection limits and function slopes for selenate
electrodes. Figures. 3, 4 show electrode functions of ISEs,
Table 1 contains LDL values and electrode function slopes.
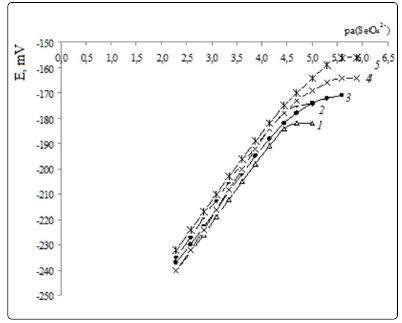
It is evident (Table 1), that all ISEs developed have neartheoretical
electrode function slopes and low LDL that decreases
further with QAS exchange center steric access improvement,
i.e. from TNODA to (oxyethyl)4 TM by 1.4 orders in absence of
TFABAHE and by 0.9 orders in presence of it. Within the QAS
series with improved steric access (from TM to (oxyethyl)4TM),
LDL decreases by 0.8 and 0.3 orders, respectively.

Selectivities of selenate ISEs. Table 2 contains lgKPot(SeO42-,
j) values for all selenate selective electrodes plasticized with
DBP. The results show that the effect of exchange center steric
accessibility increases from hydrophilic to hydrophobic anions:
while switching from TNODA to (oxyethyl)4TM brings down
lgKPot(SeO42-, SO42-) only by 0.8 orders, the same change
decreases lgKPot(SeO42-, NO3-) by as much as 5.5 orders.
Introducing TFABAHE into membranes improves
selectivity dramatically. For instance, chloride and bromide
ions show no interference with selenate. Somewhat
unexpected is the fact that some selectivity is observed in
presence of structurally similar sulfate ions; one of the possible
explanations being greater polarity of Se-O bond as compared
to S-O bond. It should be noted that introduction of TFABAHE
into selenate ISEs produces greater selectivity improvement
than for QAS-based sulfate ISEs as described in [26]. TFABAHE
content in selenate ISE membranes was in all cases 20% w/w,
because it has been shown in Reference27 that, to achieve
high selectivity toward hydrophilic anions, it is necessary to
use the neutral anion carrier in large excess to QAS.
For all ISEs, transition from (oxyethyl)2TM to (oxyethyl)4TM
the net change of lgKPot is about 0.1-0.4 orders.

The effect of plasticizer nature upon LDLs, selectivity and
function slope. Figure 5 shows electrode functions for selenate
ISEs with such plasticizers as 1-BN, BEHD and o-NPDE; LDL
values, slopes and lgKPot(SeO42-, j) are given in the Table 3.
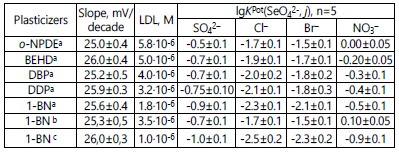
аISE based on (oxyethyl)2ТM. bISE based on ТM. c ISE
based on (oxyethyl)4ТM.
It is obvious from Table 3 that in the following series of
plasticizers o-NPDE-BEHD≈DBP-DDF-1-BN, LDLs decrease by
0.5 orders (for (oxyethyl)2ТM /TFABAHE based ISEs), while
lgKPot(SеO42-, j) - by 0.6 orders for Br-, by 0.5 orders for NO3-,
by 0.6 orders for Cl-, and by 0.4 orders for SO42-, with neartheoretical
slopes for all the ISEs studied.
A series of ISEs has been made using QASes with the enhanced
steric access to the exchange center ((oxyethyl)2 TM and (oxyethyl)4TM),
TFABAHE additive and 1-BN as a plasticizer. As shown in Table 4,
increasing the spacer length between the aromatic ring and nitrogen
atom (from TM, with no oxyethyl groups, to (oxyethyl)4TM, with the
longest chain) reduces lgKPot(SеO42-, j) by 1.0 orders for NO3-, by 0.8
orders for Br-, by 0.8 orders for Cl-, by 0.3 orders for SO42-.
The effects of the exchange center steric accessibility on
the selenate ISE selectivity can be qualitatively interpreted in
terms of the corresponding change of ion association constants
for ions being exchanged: in the model where QAS exists in the
form of ion triples with double charged anions and no associates
of greater complexity are formed, the ion association constant
kas is described by Eigen-Denison-Ramsey-Fuoss equation [28].
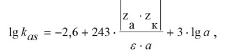
Where are charges of the anion and QAS cation being
associated; ε is the dielectric constant of the solvent; а is the closest
approach parameter for cations and anions being associated, Å.
According to [28], in close ion pairs, where no solvent
molecules are present between the associated ions, for QAS
cations and anions а parameter is about 4-7 Å. Hence, with
solvents with low or medium dielectric constant, such as DBP
(ε=6.4), DDP (ε=4.4), BEHD (ε=6) or 1-BN (ε=5) [24], the
second member of Eigen-Denison-Ramsey-Fuoss equation
makes the main contribution in kas value.
The а parameter is, at least in the first approximation, an
additive function of the anion and cation radii. It seems
obvious that the effect of QAS exchange center steric
accessibility on association constants must depend upon the
size of ions being associated. Clearly, the improvement of
steric access should lead to greater ion association constant
increase for smaller anions (r(SeO42-)=2,49Å, r(SO42-)=2.58Å,
according to [29]). When single charged anions are substituted
for double charged ones, decreasing the closest approach
parameter should considerably increase the first association
constant of a double charged anion with QAS cation. The
removal of steric hindrance for approach of the ion pair (such
as QAS+…SeO42-) to the second QAS cation should be
accompanied by increase of the second association constant
as well.
This trend for selectivity improvement is retained also in
presence of such neutral carrier as TFABAHE. It should be
noted that introduction of the neutral carrier levels both
selectivity improvement and LDL decrease effects, that can be
explained by solvate formation between anions and TFABAHE
diminishing the effect of exchanging ion size itself.
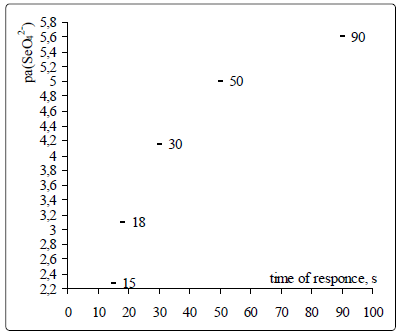
Potential drift, response time, life time. Figures 6 and 7 are
show the time dependencies for the selenate ISE with
optimized membrane composition by QAS, plasticizer and
solvating additive. The potential drift has been observed for 1
hour.
It is evident that ISE potential establishes quickly, but with
decreasing concentration (activity) the response time becomes
longer, the reason being the decreased content of main ions
in the near-electrode layer and stronger interference from
other ions.
The life time for selenate ISEs is about 3 weeks in presence
of TFABAHE and up to 9 weeks in its absence.
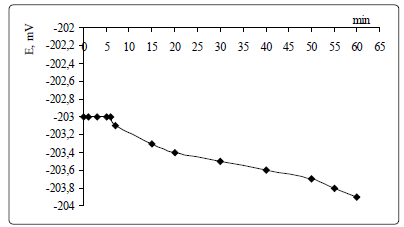
The potential drift for the ISE based on (oxyethyl)4TM, TFABAHE and 1-BN is about 0.9 mV/h, for C(SeO42-)=4.5·10-4 M.
Determination of selenate ions in model solutions with
the ISE based on (oxyethyl)4TM, TFABAHE and 1-BN was
performed by the calibration plot method and the limiting
solutions method. In all solutions, pH has been maintained at
3.2±0.1. In the limiting solutions method, two standard
solutions were used, with selenate concentrations above (C2)
and below (C1) their content in the target solution (Cx). The
calculations were done according to the formula:
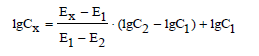
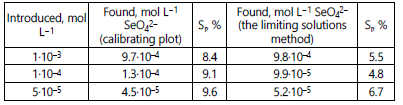
The results are presented in the Table 4.
Therefore, the limiting solutions method is preferable for
determining selenate ions in model solutions as it provides
more precision.
Conclusions
It has been established that, of all ISEs studied, the
electrode based on (oxyethyl)4TM, TFABAHE and 1-BN has
the best characteristics. Considerable LDL decrease and
selenate selectivity improvement occurs in the following QAS
series: TNODA-TB-TE-TM-BHPBTM-(oxyethyl)2TM-
(oxyethyl)3TM-(oxyethyl)4TM. Therefore, the feasibility of
«improved steric access» approach to designing ISEs for
double charged anions is confirmed.
It has been found that, in the plasticizer series o-NPDE-
BEHD≈DBP-DDF-1-BN analytical characteristics improve
considerably, that warrants recommendation of
1-bromonaphtalene as a plasticizer for selenate ISEs.
It has been shown that introducing TFABAHE as a neutral anion carrier improves selectivity of selenate ISEs considerably.
The possibility of selenate determination in model solutions
both by calibration plot method and limiting solutions
method has been demonstrated.
References
- Shlyapunova EV, Sergeev GM. Selective definition of the diverse forms of selenium in drinking waters. Vestnik N.I. Lobachevsky Nizhegor. Chemistry. 2009; 6: 96-100.
- Conde JE, Sanz AM. Selenium concentrations in natural and environmental waters. Chem. Rev. 1997; 97(6): 1979-2004. doi: 10.1021/cr960100g
- Korenovska M. Determination of arsenic, antimony, and selenium by FIHG- AAS in foods consumed in Slovakia. J. Food and Nutrition Research. 2006; 45(2): 84-88.
- Zheng J, Shibata Y, Furuta N. Determination of selenoamino acids using two-dimensional ion-pair re-versed phase chromatography with on-line detection by inductively coupled plasma mass spectrometry. Talanta. 2003; 59(1): 27-36. doi: org/10.1016/S0039-9140(02)00460-5
- Stozhko NYu, Morosanova EI, Kolyadina LI, Fomina SV. Ceramic composite electrode for the determination of selenium (IV) by the method of inversion voltammetry. Rus. J. Anal. Chem. 2006; 61(2): 158-165. doi: 10.1134/S1061934806020122
- Valencia MC, Nicolas EA, Capitan-Vallvey LF. Speciation of selenium (IV) in natural waters by solid phase spectrophotometry. Talanta. 1999; 49(4): 915-921. doi: 10.1016/s0039-9140(99)00088-0
- Kapsimali DC, Zachariadis GA. Comparison of tetraethylborate and tetraphenylborate for selenite determination in human urine by gas chromatography mass spectrometry, after headspace solid phase microextraction. Talanta. 2010; 80(3):1311-1317. doi: 10.1016/j. talanta.2009.09.022
- Ekmekçi G, Somer G. Preparation and properties of solid state selenite ion selective electrodes and their applications. Talanta. 1999; 49(1): 91-98. doi: 10.1016/S0039-9140(98)00350-6
- Ibrahim H, Issa YM, Shehab Ola R. New selenite ion-selective electrodes based on 5,10,15,20-tetrakis-(4-methoxyphenyl)-21H,23H-porphyrin- Co(II). J. Hazardous Materials. 2010; 181(1-3): 857-867. doi: 10.1016/j. jhazmat.2010.05.092
- Ekmekçi G, Somer G. A new selenite selective membrane electrode and its application. Talanta. 1999; 49(1): 83-89. doi: 10.1016/S0039-9140(98)00353-1
- Ganjali MR, Norouzi P, Faridbod F, Rezapour M, Pourjavid MR. One Decade of Research on Ion-Selective Electrodes in Iran (1996-2006). J. Iran. Chem. Soc. 2007; 4(1): 1-29.
- Antonisse MMG, Reinhoudt DN. Potentiometric Anion Selective Sensors. Electroanalysis. 1999; 11(14): 1035-1048. doi: 10.1002/(sici)1521- 4109(199910)11:14<1035::aid-elan1035>3.0.co;2-i
- Antonisse MMG, Reinhoudt DN. Neutral Anion Receptors: Design and Application. Chem.Commun. 1998; 23(4): 443-447. doi: 10.1039/A707529DGupta VK. Potentiometric sensors for inorganic anions based on neutral carrier. Arab. J. Sci. Eng. 2010; 35: 7-25.Umezawa Yо, Umezawa K, Bühlmann Ph, Nishimura Y. Potentiometric selectivity coefficients of ion-selective electrodes Part II. Inorganic anions (Technical Report). Pure Appl. Chem. 2002; 74(6): 923-994.Koseoglu SS, Lai CZ, Ferguson C, Bühlmann Ph. Response Mechanism of Ion-Selective Electrodes Based on a Guanidine Ionophore: An Apparently Two- Thirds Nernstian Response Slope. Electroanalysis. 2008; 20(3): 331- 339. doi: 10.1002/elan.200704066Bühlmann Ph, Chen Li D. Ion-Selective Electrodes with Ionophore-Doped Sensing Membranes, Supramolecular Chemistry: From Molecules to Nanomaterials. John Wiley & Sons. 2012; 5: 2539-2579.Bühlmann Ph, Pretsch E, Bakker E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998; 98(4): 1593-1688. doi: 10.1021/cr970113+Okaev EB, Stanishevsky LS. Synthesis of quaternary ammonium salts with increased steric availability of the cationic center, promising as electrode compounds. Chemical Technologies of Functional Materials: proceedings of the III rd International Russia-Kazakhstan Conference (Russian). Novosibirsk NSTU. 2017; 13-15.Okaev EB. Synthesis of new highly lipophilic quaternary ammonium salts with regulated steric accessibility of the cationic center. Proceedings of the National Academy of Sciences of Belarus. Chemistry Series. 2005; 1: 53-57.K. Kamman, Working with ionoselective electrodes (Russian). Moscow: Mir. 1980: p288.Egorov VV, Nazarov VA, Okaev EB, Pavlova TE. A new sulfate selective electrode and its use in analysis. J. Anal. Chem. 2006; 61(4): 382-388. doi: 10.1134/S1061934806040150.Matveichuk Yu, Rakhman ko E, Akayeu Ya, Stanishevskii D. Ion selective electrodes based on long-chain quaternary ammonium salts with enhanced steric accessibility, and their application for determination of hydrophilic double charged inorganic anion. Chem. Pap. 2017. doi: 10.1007/s11696-017-0320-7Dean J.A. Lange´s handbook of chemistry. McGRAW-HILL, INC.1999: p1291.Bakker E, Pretsch E, Bühlmann Ph. Selectivity of Potentiometric Ion Sensors. Anal. Chem. 2000. 72(6): 1127-1133. doi: 10.1021/ac991146nMatveychuk YuV, Okaev EB. New quaternary ammonium salts: application in ionometry Chemical Technologies of Functional Materials: proceedings of the III rd International Russia-Kazakhstan Conference (Russian). Novosibirsk NSTU. 2017; 20-23.Lomako SV, Astapovich RI, Nozdrin-Plotnitskaya OV et.al. Sulfate-selective electrode and its application for sulfate determination in aqueous solutions. Anal. Chim. Acta. 2006; 562(2): 216-222. doi: 10.1016/j. aca.2006.01.047Nazarov VA, Andronchik KA, Egorov VV, Matulis VE, Ivashkevich OA. Intramembrane complex formation study of ion selective electrode based on heptyl p-trifluoroacetylbenzoic ether. Electroanalysis. 2011. 23(5): 1058-1066. doi: 10.1002/elan.201000606Jenkins HDB, Thakur KP. Reappraisal of thermochemical radii for complex ions. J. Chen. Education. 1979. 56(9): 576-577. doi: 10.1021/ed056p576


