Research Article
Pharmacokinetic interaction study between Acyclovir and Paracetamol using HPLC-UV method
1Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Assiut University, Egypt
2Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Deraya University, Minia, Egypt
*Corresponding author: Fatma AM Abdel-aal, Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Assiut University, 71526 Assiut, Egypt, Tel: 0020-88-2411520, Fax: 0020-88-2080774, Email: famo207@yahoo.com, fatmamoustafa@aun.edu.eg
Received: July 21, 2017 Accepted: September 18, 2017 Published: September 25, 2017
Citation: Saleh GA, Askal HF, Refaat IH, Abdel-aal FAM. Pharmacokinetic interaction study between Acyclovir and Paracetamol using HPLC-UV method. Madridge J Anal Sci Instrum. 2017; 2(1): 28-34. doi: 10.18689/mjai-1000107
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Chickenpox is a highly infectious disease caused by the varicella-zoster virus. Acyclovir (ACV) is an antiviral drug that has been concomitantly used with paracetamol (PA) for its analgesic effect in the management of chicken pox infections. However, the simultaneous pharmacokinetic interaction study for ACV and PA has not yet been reported. A simple high-performance liquid chromatography assay was developed and validated for their simultaneous analysis in human urine. The proposed method presents a pharmacokinetic study for ACV with/without co-administration of PA in human urine with high precision, accuracy and sensitivity in a short time not more than 10 minutes.
Keywords: Acyclovir; Paracetamol; Human Urine; Pharmacokinetics and Drug-Drug Interaction.
Introduction
Chickenpox is highly infectious disease. Spread throughout households is very common with infection of up to 90% of vulnerable individuals who come into contact. Most infection occurs in those under 5 years of age and immunity increases with age until adulthood, immunocompromised patients and pregnant women [1]. Acyclovir (ACV), 2 - Amino - 1, 9 - dihydro - 9 - [2-(hydroxyethoxy) methyl] -6H- purin-6-one (Fig. 1) is an acycloguanosine which exhibits a selective inhibition of herpes viruses replication with potent clinical antiviral activity against the herpes simplex and varicella-zoster viruses. Therefore it is the first choice in treatment of herpes zoster (shingles), postherpetic neuralgia and chicken pox. Due to the pain associated with these diseases, patients with mild to moderate pain may respond to the over-counter analgesics such as acetaminophen (paracetamol; PA) (Fig. 1) and in severe pain it potentiates the painrelieving effects of narcotics in patients [2].
Several analytical methods have been described for the determination of ACV in pharmaceuticals and biological samples. These methods include spectrophotometric [3-8],
spectrofluorimetric [9], high performance liquid chromatography (HPLC) [10-15], capillary electrophoretic [16-18] and electrochemical methods [19-25]. However, no previous method has been described for the simultaneous determination of ACV and PA.
It was reported that ACV is taken orally and absorbed slowly and incompletely, so most of the drug is excreted unchanged in the urine [26]. Therefore there is a great need to develop a simple and sensitive HPLC method that determine ACV and make a study on its pharmacokinetics in urine. Therefore, the present work is designed to develop an accurate and precise analytical method to determine ACV in human urine in case of concomitant administration of PA. The developed method is useful to explore the impact of PA administration on the pharmacokinetic behavior of ACV in human. It is also useful to explore the expected drug-drug interaction between ACV and PA which may affect the required ACV dose especially in immunocompromised patients, children, pregnant woman and patients with renal impairments in whom PA is the safest analgesic drug.
Experimental
Reagents and chemicals
ACV was kindly supplied by (Global Napi Pharmaceuticals,
6th October, Egypt), PA was supplied by (CID pharmaceutical company, Assiut, Egypt) and guanine used as an internal standard (IS) was purchased from (Sigma Co., Cairo, Egypt).
Phosphoric acid, methanol and acetonitrile were obtained from (Sigma Co., Cairo, Egypt). All other chemicals and solvents used in this work were of HPLC analytical grade.
Human urine samples were obtained from five female healthy volunteers at Assiut city.
Apparatus and chromatographic conditions
A Younglin Autochro-3000 HPLC system (Younglin,
Dongan-Gu, South Korea) with a UV detector, a Rheodyne injection valve with a 20 μl loop was used. The analytical column employed was C18 reversed phase column of 250
× 4.6 mm ID, 5 μm dimensions (Supelco, Bellefonte, PA, USA).
The mobile phase was comprised of methanol: water adjusted to pH 4.0 with phosphoric acid (1%) (15:85, v/v). The mobile phase was prepared freshly, filtered and degassed by sonication before use. All separations were performed isocratically at a flow rate of 1.0 ml min-1 at ambient temperature. The detector was operated at 254 nm. For the protection of the analytical column during working with urine samples, C18 (4 mm×3 mm i.d.) security guard cartridge system (Phenomenex, Torrance, CA, USA) was used.
Preparation of standard solutions
Stock solutions of each drug and IS (1.0 μg/ml) were prepared by dissolving the accurately weighed ACV in bidistilled water, PA in methanol and IS (Fig.1) in 0.1 M Hydrochloric acid (HCl). Aqueous stock solutions were stored at 4°C in well-closed light resistant containers. The working solutions were prepared by diluting the stock solution with the mobile phase to a final concentration and prepared immediately before their use.
Preparation of calibration solutions in human urine
Standard calibration solutions were prepared by spiking drug-free human urine with stock ACV and PA standard solutions and IS solutions, which were then further diluted with the mobile phase (diluted with the ratio of 1:100) to achieve final concentrations from 0.5 to 20.0 μg/ml for ACV and from 1.0 to 20.0 μg/ml for PA with a final concentration of 2.0 μg/ml from IS solution.
Validation of the method
The validation process was carried out according to Guidance for Industry - Bioanalytical Method Validation,
recommended by the US Food and Drug Administration
[27], ICH guidelines [28] and British pharmacopoeia [29].
Linearity and limits of detection and quantification
Linearity was assessed by six-point calibration curves in standard solutions and by spiking known amounts of each drug and IS to human urine samples five times on three consecutive days. The curves were constructed by plotting the peak area ratio of each drug to the IS versus the concentration. The evaluated concentration ranged from 0.2 to 20.0 μg/ml for each drug in their standard solutions and from 0.5 to 20.0 μg/ml for ACV and from 1.0 to 20.0 μg/ml for PA in human urine samples. The curves were evaluated by linear regression. The limit of detection (LOD) was established as the lowest concentration of the analyte that can be detected but not necessarily quantified and calculated at signal-to-noise (S/N)
ratio of 3. Whereas, the limit of quantitation (LOQ) was established as the lowest concentration of an analyte that can be quantified with good precision and accuracy and calculated at S/N ratio of 10.
Accuracy and precision
To evaluate the precision and accuracy of the method in standard solutions and human urine samples, three concentrations including lower, middle, and upper limits of each calibration curve of each drug in five replicates were analyzed in three different days according to FDA [27] and ICH [28]. Intra-day and inter-day precision were calculated and expressed as coefficient of variation (CV). The intraday and inter-day accuracy were calculated as the percentage of the calculated concentrations compared to the true concentrations.
Robustness
For the determination of the proposed method′s robustness, mobile phase ratio, mobile phase pH, detection wavelength and flow rate were varied within a realistic range,
and the quantitative influences of the variables were determined.
Selectivity
Selectivity was determined by comparing the peaks of the studied drugs with peaks of another co-administered drugs as chloropheniramine (ChP) or structurally related drugs as valacyclovir (VAL) to determine if these drugs interfere with the studied drugs or not.
Recovery and extraction efficiency
The efficiency of extraction of ACV and PA from s spiked human urine was measured by analyzing three concentrations
(low, middle and high) of the samples. The drug recovery was determined by comparing peak areas obtained from the spiked samples after extraction to the standard solution at the same concentration of the spiked samples. The relative recovery was calculated by comparing the concentrations obtained from the drug supplemented human urine with actual added amounts.
In vivo Application of the developed analytical method on human urine
A pharmacokinetic study was performed in five healthy female volunteers aged between 25 to 30 years and weighing from 70 to 85 kg. The volunteers were instructed to abstain from taking any medication at least 2 weeks prior to and during the study period. The protocol was the conventional,
two-way, crossover study with ten subjects and a one-week washout period. In the first trial period, after an overnight fasting, volunteers were given a single oral dose of 400 mg ACV from (Glaxo SmithKline Co., Cairo, Egypt) (Zovirax suspension contain 400mg ACV/ 5ml) with 200 ml of water.
In the second trial period and after and a one-week washout period, volunteers were given a single oral dose of
400 mg ACV (Zovirax suspension) and 500 mg of PA (Paramol tablets from CID Company, Assiut, Egypt). In the two cases the drugs were administered after overnight fasting and urine samples were collected at 0.0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0,
9.0 and 12.0 hrs after drug administration. The samples were collected and stored at 4- °C until use. The pharmacokinetic parameters of ACV were calculated by non compartmental analysis using WinNonlin (version 3.3; Pharsight Corp.,
Mountain View, CA). The investigated pharmacokinetic parameter included; the maximum urine concentration (Cmax) and the maximum urine concentration time (tmax) were directly obtained from the raw data. The area under the urine concentration-time curve (AUC0-t) up to the last measurable time point (12.0 hrs in this study) and to infinity (AUC0-∞) were obtained. Kel represents the apparent terminal rate constant.
MRT represented the maximum residual time. The half-life of drug elimination (t1/2) was calculated as 0.693/ Kel.
Results and Discussion
Development of HPLC method Mobile Phase optimization
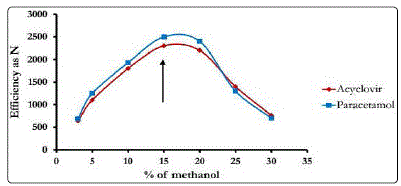
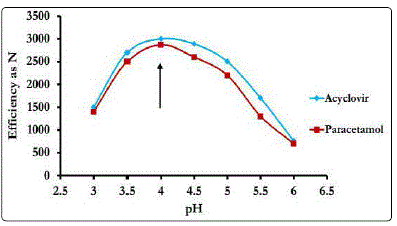
The effect of organic modifier (acetonitrile, methanol and their mixture) on peak resolution and peak symmetry was investigated. Use of acetonitrile as the organic modifier in the mobile phase did not result in adequate sensitivity, selectivity and peak symmetry. However, methanol as organic component resulted in better sensitivity and symmetrical peaks but variation in the amount of methanol in the mobile phase affected resolution and runtime. The optimum results were found using 15% methanol as shown in Fig 2. Also pH of the medium is a critical factor in the resolution of the studied mixture. In order to better distinguish between matrix components, ACV, PA and IS, the pH value of the mobile phase was optimized. It was found that the optimum pH for the separation of the studied drugs is 4.0 as shown in Fig 3.
Hence, an elution consisting of methanol and water (adjusted to pH 4.0 with phosphoric acid) (15:85, v/v) was employed.
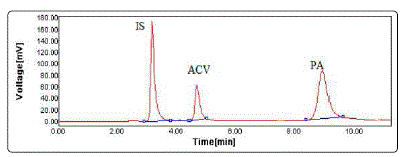
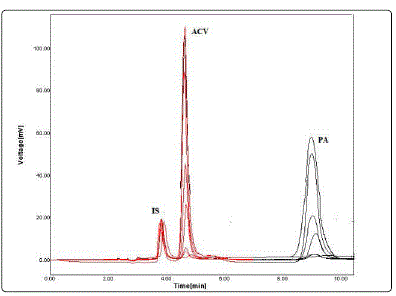
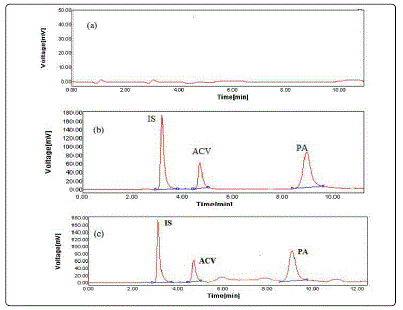
Fig. 4 shows a typical chromatogram of standard solutions of the studied drugs and IS in the mobile phase. The retention times were about 3.2 min for IS, 4.7 min for ACV and 9.2 min for PA.
Effect of flow rate on column efficiency
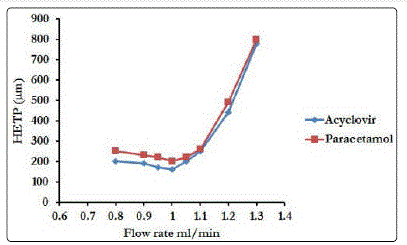
The influence of the mobile phase flow rate on the performance of the column for the separation of tested mixture was investigated. The flow rate was varied in the range 0.8-1.3 ml/min. The Van Deemter plot (height equivalent to a theoretical plate HETP versus flow rate) of the tested mixture is shown in Fig. 5 that revealed the effect of the flow rate on the column efficiency for separation of the tested mixture. As can be seen, the efficiency of the column is better and low pack pressure at flow rate of 1.0 ml/min, so it was chosen for further experiments.
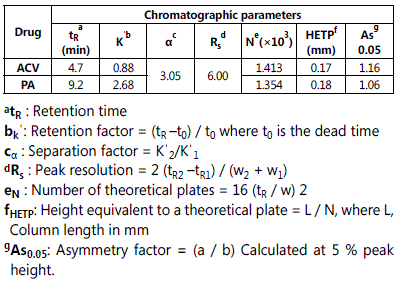
System suitability
System suitability tests were investigated in accordance with the BP guidelines [29], to ensure adequate performance of both the chromatographic system and the equipment for the analysis to be performed. Different chromatographic parameters were calculated from the experimental data, including the retention time (tR), retention factor (kˈ), separation factor (α), peak resolution (Rs), number of theoretical plates (N), height equivalent to a theoretical plate (HETP), and the asymmetry factor (As0.05). All these parameters are usually employed in assessing the performance of the column. Results obtained from system suitability are presented in Table 1. Good agreement was found when results were compared with the recommendedvalues [29].
Selection of detection wavelength
The studied drugs were detected at different wavelengths;
220, 242 and 254 nm to determine the most proper one. The best sensitivity was obtained at a wavelength of 254 nm, so it was selected as the wavelength of choice for our developed chromatographic method.
Optimization of the sample pretreatment procedure
According to human urine samples, dilution step was enough without further extraction techniques. The recoveriesof ACV and PA from human urine were examined by comparing peak areas ratios (peak area of sample/peak area of IS) of spiked plasma samples or spiked humanurine Samples with peak areas ratios obtained from the same amounts of unextracted ACV and PA mixtures in the mobile phase.
Method validation
Linearity and sensitivity
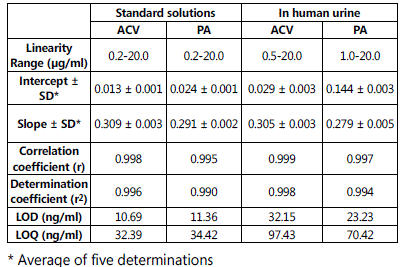
Under the optimal conditions, the calibration curves for ACV and PA in the mobile phase and human urine spiked with ACV and PA were prepared by plotting the peak areas ratio of ACV and PA to IS vs the concentration in μg/ml. Fig.6 shows over layed chromatograms illustrating the calibration curves of ACV and PA binary mixtures. The parameters of the calibration curves for the proposed method are summarized in Table 2. LOD and LOQ were calculated at S/N ratios of 3
and 10, respectively. The proposed method is sensitive enough for drug monitoring and pharmacokinetic studies. In comparison with the reported methodologies for analysis of the individual drugs, the sensitivity of the proposed methodology is higher than some of the reported methods
[33-35], however, it should be taken into account that in this case two drugs were simultaneously determined
Precision, accuracy and recovery
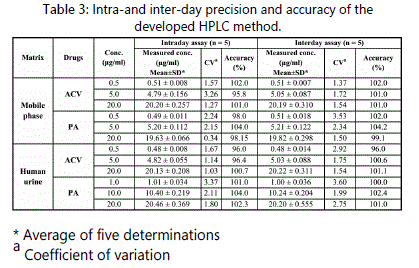
Repeatability and intermediate precision of the developed method were expressed in terms of coefficients of variation (CV)
of the peak area. The results showed that intra- and inter-day variations at three different concentrations (lower, medium and higher) for all the tested compounds in their standard solutions and human urine were within the acceptable range. The CV for both the inter-day and intraday precision of the method were found to be not more than 3.53 % for both drugs in their standard solutions and 3.60 in spiked human urine as shown in Table 3. Accuracy in human urine was determined by comparing the measured concentrations of ACV and PA in human urine with actual values and expressed as percentage. The recoveries were found between 95.8 and 104.2% indicating good accuracy.
The obtained accuracy and precision was satisfactory for quality control measurements for the examined compounds. In comparison with reported methods [33-35], the obtained recoveries were much better than those obtained by most of the reported methods.
Robustness
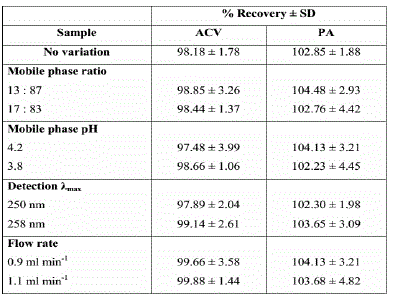
The study involved calculations of small variations in the method parameters including; mobile phase composition ratio, mobile phase pH, detection wavelength and the flow rate. The data were presented as percentage recovery in Table 4. None of these variables had a significant effect on the determination of investigated drugs.
Therefore, the developed HPLC method was considered robust.
Selectivity
No interference from endogenous urine constituent was observed also at the retention times of the studied drugs and the internal standard as shown in Fig. 7.
Pharmacokinetics of ACV in human urine
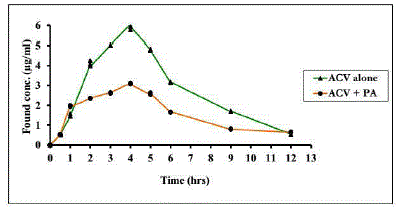
This method has been applied to the determination of ACV in human urine following single oral administration of 400 mg ACV with/without 500 mg PA in five healthy volunteers. Fig. 8
shows a typical urine concentration - time profile for both cases.
Resulted pharmacokinetic parameters are shown in Table 5.
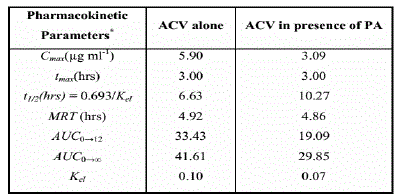
Cmax: Maximum urine concentration.
tmax: Maximum urine concentration time.
AUC0-t: Area under the urine concentration- time curve up to
the last measurable time point (12 hrs in this study).
AUC0-∞: Area under the urine concentration- time curve to infinity.
Kel: Apparent terminal rate constant.
MRT: Maximum residual time.
t1/2:Half-life of drug elimination.
Clinical importance of the pharmacokinetic studies on ACV with/without PA
As reported before [26], ACV was excreted in high percentage unexchanged in urine, so in this method a pharmacokinetic study was applied on urine samples collected at different times after single administration of ACV dose with or/without PA.
Coadministration of PA with ACV is commonly in viral infections in children or pregnant women for treatment of herpes simplex and chicken pox. These groups of people are very sensitive and require highly accurate dose especially that antiviral drugs such as ACV is a very important drugs in their treatment. By inspection of Fig. 8 and Table 5, the results revealed that the newly developed analytical method has the required sensitivity to characterize the pharmacokinetic behavior of ACV with/without PA coadministration. The decrease in the pharmacokinetic parameters in the second set (ACV with PA) rather than the first set (ACV alone) had depicted the decreased excretion of unchanged due to increased absorption of ACV upon coadministration of PA. This might be attributed to either their in-vivo interaction causing synergistic effect or activation of ACV absorption [36]. Hence administered ACV dose should be adjusted upon coadministration of PA to get the required blood concentration.
Conclusion
A simple, fast and efficient reversed phase HPLC method was developed for the determination of acyclovir that coadministered with paracetamol in the treatment of chicken pox infections. Validation study results have shown that the developed method was precise, accurate, selective and robust to meet the requirements of FDA and ICH guidelines. To the best of our knowledge, this is the first HPLC method for the simultaneous determination of ACV and PA. The selectivity of the method enabled its successful application for the routine analysis of the studied drugs in biological samples. In addition,
the relatively short run time required for analysis, as well as the good sensitivity and reliability contributed to the success of the method when it was applied for the pharmacokinetic study of ACV in human urine after a single administration of ACV with/without PA co-administration.
Conflict of Interest:
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
References
- Vyse AJ, Gay NJ, Hesketh LM, Morgan-Capner P, Miller E. Seroprevalence of antibody to varicella zoster virus in England and Wales in children and young adults. Epidemiol. Infect. 2004; 132(6): 1129-1134.
- Sweetman Eds SC. Martindale, The Complete Drug Refernce. Pharmaceutical Press, London, 33 ed., pp. 862 - 864. 911. 2002
- Basavaiah K, Prameela HC. Simple spectrophotometric determination of acyclovir in bulk drug and formulations. Farmaco. 2002; 57(6): 443-449. doi: 10.1016/S0014-827X(02)01237-5
- Ayad MM, Abdellatef HE, El-Henawee MM, El-Sayed H.M. Spectrochimica Acta A Molecular Biomolecular Spectroscopy. 2007; 66: 106-110.
- Pant PP, Felice SVS, Gurung SCBV, Divya G, N RM. J. Appl. Chem. Res. 2009; 10. 7-12.
- Reddy SA, Chakraborty R, Sen S, Parameshappa B. Spectrophotometric determination and validation of Acyclovir. Archives of Applied Science Research. 2011; 3(1): 328-332.
- Dongare US, Chemate SZ, Jadhav SA, Pawar VR. Spectrophotometric Determination And Validation Of Acyclovir In Tablet Dosage Form. Int. J. Pharm Tech Res. 2012; 4(4): 1840-1845.
- Ajima U, Onah JO. Journal of Applied Pharmaceutical Sciences. 2015; 5: 65-69.
- Darwish IA, Khedr AS, Askal HF, Mahmoud RM. Simple fluorimetric method for determination of certain antiviral drugs via their oxidation with cerium (IV). Farmaco. 2005; 60 (6-7): 555-562.
- Loregian A, Gatti R, Palu G, De Palo EF. Separation methods for acyclovir and related antiviral compounds Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences. 2001; 764(1): 289-311.
- Basavaiah K, Prameela HC, Chandrashekar U. Simple high-performance liquid chromatographic method for the determination of acyclovir in pharmaceuticals. Farmaco. 2003; 58(12): 1301-1306. doi: 10.1016/S0014- 827X(03)00157-5
- Huidobro AL, Ruperez FJ, Barbas. LC methods for acyclovir and related impurities determination. J of Pharmaceut Biomed Analysis. 2005; 37(4): 687-694. doi: 10.1016/j.jpba.2004.11.039
- Sasanya JJ, Abd-Alla AM, Parker AG, Cannavan A. Analysis of the antiviral drugs acyclovir and valacyclovir-hydrochloride in tsetse flies (Glossina pallidipes) using LC-MSMS. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences. 2010; 878(26): 2384- 2390. doi: 10.1016/j.jchromb.2010.07.008
- Shao C, Dowling TC, Haidar S, Yu LX, Polli JE, Kane MA. J. of Anal. Bioanal. Tech. 2012; 3: 1-6.
- Dewulf J, Galanti L, Godet M, Gillet P, Jamart J. Hecq JD. Long-term stability of acyclovir in 0.9% NaCl infusion polyolefin bags at 5 ± 3 ° C after freeze-thaw treatment. Annales Pharmaceutiques Françaises. 2015; 73: 108-113. doi: 10.1016 / j.pharma.2014.10.003
- Zhang S, Yuan Z, Liu H, Zou H, Xiong H, Wu Y. Analysis of acyclovir by high performance capillary electrophoresis with on-column amperometric detection. Electrophoresis. 2000; 21: 2995-2998. doi: 10.1002/1522-2683(20000801)21:14<2995::AID-ELPS2995>3.0.CO;2-P
- Vo HC, Henning PA, Leung DT, Sacks SL. Development and validation of a plasma assay for acyclovir using high-performance capillary electrophoresis with sample stacking. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences. 2002; 772 (2): 291-297. doi: 10.1016/S1570-0232(02)00116-2
- Leung DT, Henning PA, Wagner EC, Blasig A, Wald A, Sacks SL, et at. Inadequacy of Plasma Acyclovir Levels at Delivery in Patients With Genital Herpes Receiving Oral Acyclovir Suppressive Therapy in Late Pregnancy. Journal of obstetrics and gynaecology Canada. 2009; 31(12): 1137-1143. doi: 10.1016/S1701-2163(16)34374-2
- Wang F, Chen L, Chen X, Hu S. Studies on electrochemical behaviors of acyclovir and its voltammetric determination with nano-structured film electrode. Anal. Chim. Acta. 2006; 576(1): 17-22. doi: 10.1016/j. aca.2005.12.023
- Shetti NP, Malode SJ, Nandibewoor ST. Electrochemical behavior of an antiviral drug acyclovir at fullerene-C(60)-modified glassy carbon electrode. Bioelectrochemistry. 2012; 88: 76-83. doi: 10.1016/j.bioelechem.2012.06.004
- Castro AA, Cordoves AIP, Farias PAM. Determination of the Antiretroviral Drug Acyclovir in Diluted Alkaline Electrolyte by Adsorptive Stripping Voltammetry at the Mercury Film Electrode. Analytical Chemistry Insights. 2013; 8: 21-28. doi: 10.4137/ACI.S11608
- Shaidarova LG, Gedmina AV, Zhaldak ER, Chelnokova IA, Budnikov HK. Amperometric Detection of Allopurinol in Flow-Injection Analysis on Electrodes Modified by Carbon Nanotubes and Mixed-Valence Ruthenium and Iridium Oxides. Journal of Pharmaceutical Chemistry. 2015; 48(8): 749- 754. doi: 10.1007/s11094-014-1146-z
- Shpigun LK, Andryukhina EY, Protasov AS. Sequential injection-adsorptive stripping voltammetric quantitation of purine nucleobases using an electrochemically activated carbositall electrode. Journal of Electroanalytical Chemistry. 2015; 743: 46-52. doi: doi.org/10.1016/j.jelechem.2015.02.025
- Sadikoglu M, Saglikoglu G, Yagmur S, Orta E, Yilmaz S. Current Analytical Chemistry. 2015; 7: 130-135.
- Saleh G, Askal HF, Refaat IH, Abdel-aal F. Adsorptive Square Wave Voltammetric Determination of Acyclovir and Its Application in a Pharmacokinetic Study Using a Novel Sensor of β-Cyclodextrin Modified Pencil Graphite Electrode. Bull. Chem. Soc. Japan. 2015; 88: 1291-1300. doi: 10.1246/bcsj.20150112
- Beale JM, Block JH. Wilson and Gisvold′s Textbook of Organic, Medicina and Pharmaceutical Chemistry. Eds. Lippincott Williams & Wilkins, London, 12th ed., pp. 340 - 341. 2000.
- Food Drug Administration, Guidance for Industry: Bioanalytical Method Validation, Center for Drug Evaluation and Research. Rockville. 2001.
- International Conference on Harmonization (ICH) Topic Q2 (R1): Validation of Analytical Procedure: Text and Methodology. 2005.
- The British Pharmacopoeia, The Stationary Office, London. 2016. vol II and III, 61-62, 2268 - 2271.
- Bangaru RA, Bansal YK, Rao AR, Gandhi TP, Rapid. simple and sensitive high-performance liquid chromatographic method for detection and determination of acyclovir in human plasma and its use in bioavailability studies. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences. 2000; 739(2): 231-237. doi: 10.1016/S0378- 4347(99)00488-0
- Teshima D, Otsubo K, Yoshida T, Itoh Y, Oishi R. A simple and simultaneous determination of acyclovir and ganciclovir in human plasma by highperformance liquid chromatography. Journal of Biomedical Chromatography. 2003; 17(8): 500-503. doi: 10.1002/bmc.258
- Tzanavaras PD, Themelis DG. High-throughput HPLC assay of acyclovir and its major impurity guanine using a monolithic column and a flow gradient approach. Journal of Pharmaceutical Biomedical Analysis. 2007; 43(4): 1526-1530. doi: 10.1016/j.jpba.2006.11.002
- Sun S, Liu G, Wang Y. Simultaneous Determination of Acetaminophen, Caffeine, and Chlorphenamine Maleate in Paracetamol and Chlorphenamine Maleate Granules. Chromatographia. 2006; 64(11): 719-724.
- Palabıyık M, Onur F. The Simultaneous Determination of Phenylephrine Hydrochloride, Paracetamol, Chlorpheniramine Maleate and Dextromethorphan Hydrobromide in Pharmaceutical Preparations. Chromatographia. 2007; 66: S93-S96.
- Emami J, Bazargan N, Ajami A. Research in Pharmaceutical Sciences. 2009; 4: 47-54.
- European Medicines Agency, Guideline on the investigation of drug interactions. 2012.


