Research Article
Chemical shift at the X-ray K-absorption edge of Zn in some Zn compounds
1Nuclear Physics Division Bhabha Atomic Research Centre, Mumbai, India
2Atomic & Molecular Physics Division Bhabha Atomic Research Centre, Mumbai, India
*Corresponding author: Daisy Joseph, Nuclear Physics Division, Bhabha Atomic Research Centre, Bombay University, Mumbai, India, Email: djoseph@barc.gov.in
Received: February 17, 2017 Accepted: March 16, 2017 Published: March 22, 2017
Citation: Joseph D, Patra N. Chemical shift at the X-ray K-absorption edge of Zn in some Zn compounds. Madridge J Anal Sci Instrum. 2017; 2(1): 25-27. doi: 10.18689/mjai-1000106
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
It is known that the X-ray absorption edge of metal ion may change to different extent depending upon the chemical environment viz. effective charge, nature of ligands, co-ordination numbers, electronegativity of anions, covalent character of the bonds, surrounded the metal ion, even at the same oxidation state. This change in the absorption energy of the metal ion in its compound from the pure metallic state (zero oxidation state) is known as the chemical shift ΔE (eV) of the metallic ion. In the present study by means of synchrotron based X-ray absorption spectroscopy study we have calculated the chemical shift and their corresponding effective charge on the Zn atoms in different Zn compounds where the Zn is present in the 2+ oxidation state.
Keywords: X-ray Absorption Edge; Chemical Environment; Synchrotron; Zn; Oxidation State.
Introduction
The study of the chemical shift in the compounds has been carried by measuring the K absorption edge of the Zn in the Energy Scanning EXAFS beamline (BL-09) at Indus-2
Synchrotron source (2.5 GeV, 100 mA) at the Raja Ramanna Centre for Advanced Technology (RRCAT), Indore, India [1-2].
Experimental Details
The beam line operates in the photon energy range of 4-25 KeV. The beamline optics consists of an Rh/Pt coated collimating meridional cylindrical mirror used for the collimation of the beam. The collimated beam from the pre-mirror is monochromatized by a Si (111) (2d=6.2709) based double crystal monochromator (DCM). The mirror prior to the DCM is used for the higher harmonic rejection. Second sagittally bent cylindrical crystal of the DCM is used for horizontal focusing of the beam while another Rh/Pt coated bendable post mirror facing down is used for vertical focusing of the beam at the sample position. The measurements at the Zn edge have been carried out at the transmission mode using two ionization chambers of 30 cm length each. Proper gas mixture was used to have 10-20% absorption in the 1st first ionization chamber and 70-90% absorption in the 2nd ionization chamber. The 1st ionization chamber measures the incident flux (I0) and the 2nd ionization chamber measures the transmitted flux (It). The X-ray absorption co-efficient is determined as μ = ln(I0/It). For the measurement the fine powder of the samples were palletized to 15 mm diameter and were kept in a kapton tape. Finally the experimentally obtained data was calibrated with the standard metal foil and normalized by the ATHENA subroutine of the IFEFIT software package [3].
Results and Discussion
Fig.1. shows the XANES spectra of the samples of various Zn compounds along with that of Zn metal foil while the 1st order derivative spectra are shown in the inset of fig.1.
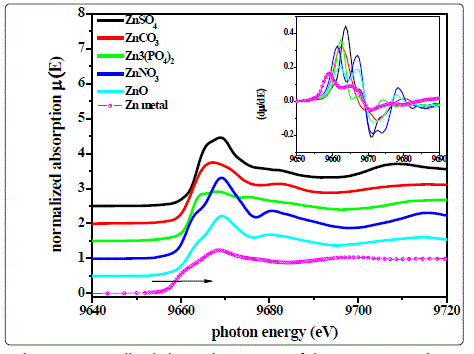
Figure 1. Normalized absorption spectra of the Zn compounds at Zn K-absorption edge (Inset is the 1st order derivative of the absorption spectra)
The 1st inflection points in the μ(E) versus E curves or the peak positions in the derivative spectra indicate the K-absorption edge (core 1s orbital) of the Zn atoms in the compounds. From the figure it can be clearly observed that the absorption edges of the Zn atoms shifted toward right in comparison to the pure metallic Zn (Zn0), indicating a chemical shift in the samples having different kinds of ligands attached to it. The chemical shift is defined as: ΔE = Ec(Compound) - Em(metal) (1) The values of the chemical shifts of the compounds studied have been summarized in the Table-1
Table 1. Chemical-shift and Effective charge at the Zn K-edge of different Zn compounds
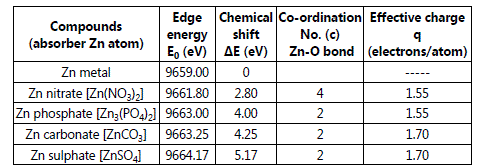
The result shows that for all the samples there is a positive shift of the absorption edges from the pure metallic phase. This trend is attributed to the successive increase in the electronegativity of the ligands and causes a decrease in the covalent character of the bonds in the compounds [4]. Thus all the above mentioned factors involve in redistribution of the valance electrons of the constituent atoms and produce an effective nuclear charge on the metal ion which causes the shift in the absorption energy. Several empirical relations have been propped in the literature to calculate the effective charge on the metal ion in binary and ternary compounds. Among those the methods propped by Suchet [5] and Pauling [6] are mostly used methods, which have been widely used to calculate the effective charge on cations in the binary and ternary compounds [6-8]. According to Suchet′s method [5], the effective charge on a cationic species involved in formation of a chemical bond is given by:
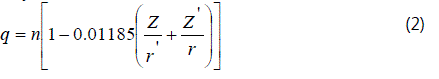
where Z, - total number of electrons r- the ionic radius and n -oxidation number of the cation and Z′- total number of electrons in the anion and r′ -the ionic radius in the anion.
Pauling [6] has alternatively represented an empirical relation of effective charge as:

Where n is the valance of the absorbing cation and I be the iconicity of the metal-ligand bond which is given as:
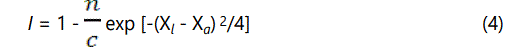
Where Xl and Xa are the electronegativities of the ligand and the absorbing atom, respectively, and c is the coordination number of the absorbing atom. This expression of ionicity is found to be agreeing with the experimentally obtained electric dipole moment of standard molecules. However it has been observed that by other worker [9,10] that the above mentioned relation is not suitable to calculate the effective charge in the compound having anionic radicals like NO3-, SO42-, CO32-, PO43- etc. For complex samples viz., Ammonium Diuranate and Uranyl Nitrate Hexahydrate, as we have shown in earlier communication [11], the effective charges can be calculated approximately by considering the first coordination shell around the absorbing atom. We have estimated the effective charges on Zn in the studied compound by considering the first oxygen coordination shell, which is tetrahedral in case of Zn nitrate and phosphate and octahedral in case of carbonate and sulphate compounds and using Pauling′s formalism described above.
Conclusion
The values of the effective charges of Zn in these compounds have also been shown in Table-1 along with the corresponding chemical shift (ΔE) values obtained from XANES data, which shows an increase in chemical shift with increase in the effective charge as expected.
Acknowledgements
The authors wish to thank Dr Alok Saxena, Head,NPD, Dr Dibyendu Bhattacharya ,AMPD and Dr N.K.Sahoo ,AD,Physics Group for their support and guidance and Shri Harpreet Singh Kainth for providing the samples.
References
- Basu S, Nayak C, Yadav AK, Agrawal A, Poswal AK, Bhattacharyya D, et al. A comprehensive facility for EXAFS measurements at the INDUS-2 synchrotron source at RRCAT. Indore. India. J. Phys. Conf. Ser. 2014; 493(1): 12-32.
- Poswal AK, Agrawal A, Yadav AK, Nayak C, Basu S, Kane SR, et al. Commissioning and First Results of Scanning Type EXAFS Beamline (BL- 09) at INDUS- 2 Synchrotron Source. AIP Conf. Proc. 2014; 1591(1): 649. doi: 10.1063/1.4872706
- Newville M, Ravel B, Haskel D, Rehr JJ, Stern EA, Yacoby Y. Analysis of multiple-scattering XAFS data using theoretical standards. Physica B. 1995; 208(209): 154-156. doi: 10.1016/0921-4526(94)00655-F
- Chetal AR, Mahto P, Sarode PR. Chemical-shift of the X-ray k-absorption edge of co in some compounds, complexes and minerals. J. Phys. Chem. Solids. 1988; 49(3): 279-283. doi: 10.1016/0022-3697(88)90079-0
- Suchet JP. Chemical physics of semiconductors. Van Nostrand Publisher, 1965.
- Pauling L. The nature of chemical bond. Cornell Univ. Press Publisher, 1960.
- Joseph D, Basu S, Jha SN, Bhattacharyya D. Chemical shifts of K X-ray absorption edges on copper in different compounds by X-ray absorption spectroscopy (XAS) with Synchrotron radiation. Nuclear Instruments and Methods in Physics Research B. 2012; 274: 126-128. doi: 10.1016/j. nimb.2011.12.011
- Joseph D, Yadav AK, Jha SN, Bhattacharyya D. Chemical shift of Mn and Cr K-edges in X-ray absorption spectroscopy with synchrotron radiation. Bull. Mater. Sci. 2012; 36(6): 1067-1072.
- Joseph D, Nayak C, Venu Babu P, Jha SN, Bhattacharyya D. Chemical shift of U L3 edges in different uranium compounds obtained by X-ray absorption spectroscopy with synchrotron radiation. Bull. Mater. Sci. 2014; 37(3): 643-647.
- Nigam AK, Gupta MK. X-ray K absorption edge of copper in some of its compounds. J. Phys. F. Metal Phys. 2014; 4(7): 1084.
- Ballal MM, Mande C. X-ray spectroscopic study of some chalcopyrites. J. Phys. Chem. Solid. 2014; 11(4): 38.


