2nd International Conference on Petrochemistry
April 25-27, 2018 Rome, Italy
Effect of Si/Al Ratio and Metal Content of the Catalyst on Non-Oxidative Conversion of Natural Gas into Value Added Chemicals
Department of Chemical Engineering, IIT Delhi, India
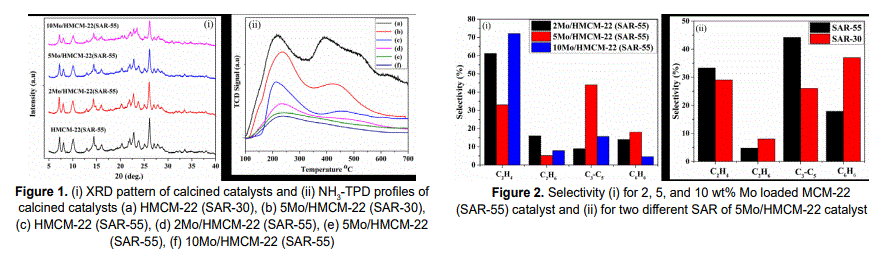
Natural gas has emerged as an attractive key feedstock for the production of liquid fuels and commodity chemicals. Major challenge in natural gas conversion comes with the methane activation as well as C2 and higher coupling reactions. Methane is a well-known highly stable light alkane with tetrahedral geometry having sp3 hybridization and exhibits highest C-H bond dissociation energy (439kJ/mole). In terms of molecular orbital chemistry, the difficulties in the activation of methane can be understood by the level of lowest unoccupied molecular orbital (LUMO) which is high and that of highest occupied molecular orbital (HOMO) which is low and thus it is difficult to exchange electrons from HOMO and LUMO. However, various methods are available in heterogeneous catalysis for methane conversion process, in which direct non-oxidative methane conversion into higher hydrocarbons is a potential approach using zeolite (HZSM-5/HMCM-22) supported molybdenum (Mo) catalyst. Catalyst preparation and its characterization plays a crucial role in the said process and the factors such as metal loading, acidity, porosity and framework structure of the catalyst significantly affect the catalytic performance. In this regard, molybdenum loading effect over zeolite support (HMCM-22), SiO2/Al2O3 (SAR) effect of the support varying the acidity of the catalyst, and also reaction parameters have been studied for the process. Investigations revealed that molybdenum loading does not affect the channel framework of the HMCM-22 zeolite as confirmed by XRD pattern of the calcined catalysts, Figure. 1(i), however crystallinity decreases slightly at higher loading. Acidity of the catalyst was determined by NH3-TPD and it was inferred that the acidity decreases on Mo loading over the HMCM-22 zeolite due to migration of Mo species into zeolite (HMCM-22) channels as confirmed by Figure. 1(ii). In the catalyst activity study, effect of Mo loading and SAR effect of HMCM-22 was tested at 700°C temperature under atmospheric pressure and 720 mL/g.h GHSV and it was observed that comparatively 5 wt% Mo loading is optimum for methane conversion into higher hydrocarbons (C6H6 selectivity 18%) as shown in Figure. 2(i). It was also concluded that, the selectivity of aromatics (benzene) increases up to to 37% with SAR-30 as compared with SAR-55 (18%) as shown in Figure. 2 (ii). In conclusion, 5 wt% Mo laoding, lower SAR (30) of zeolite support are effective for the catalyst design in the said process.
Biography:
Prof. K. K. Pant has more than 25 years of academic and industrial research experience having 125+ publications and 5 patents besides more than 150 in conference proceedings. He received his PhD degree in chemical Engineering from IIT Kanpur, 1997 and currently working as Petrotech Chair Professor in the department of chemical engineering IIT Delhi. Major research interests are heterogeneous green catalysis, reaction kinetics, hydrocarbon conversion processes, green technologies for sustainable energy and environment, biomass conversion, biofuel, fuel from waste biomass and waste plastic, metal recovery from WEEE waste, integrated bio-refinery, clean energy and bio-renewable energy. In addition, he successfully completed 20 high impact projects and consultancies valued more than 6 million USD from Indiaʼs and worldʼs top most premier companies and organization such GAIL, HPCL, DRDO, NOVOD, MHRD, CSIR, IARI, Ministry of Defence, Ministry of Fertilizers, DST Government of India and Petrochemical Society of India.



