Research Article
Preparation of PVC-NiFeCo3-Hybrid membranes for the Pervaporation Separation of Toluene-Heptane mixtures
1Laboratory of Polymer Chemistry (LCP), University of Sciences and Technology Oran Mohamed Boudiaf (USTO.MB), B.P. 1505 El Menaouer, Oran 31000, Algeria
2Laboratory of Physics-Chemistry of Materials (L.P.C.M): Catalyse and Environment, University of Sciences and Technology Oran Mohamed Boudiaf (USTO.MB), B.P. 1505 El Menaouer, Oran 31000, Algeria
*Corresponding author: Leila Aouinti, Laboratory of Polymer Chemistrry (LCP), University of Sciences and Technology, Oran Mohamed Boudiaf (USTO.MB), B.P. 1505 El Menaouer, Oran 31000, Algeria, E-mail: lalaaouinti@yahoo.fr
Received: February 26, 2019 Accepted: March 18, 2019 Published: March 25, 2019
Citation: Aouinti L, Boukraa FDS, Chirane S, Arab SM. Preparation of PVC-NiFeCO3-Hybrid membranes for the Pervaporation Separation of Toluene-Heptane mixtures. Int J Petrochem Res. 2019; 3(1): 269-273. doi: 10.18689/ijpr-1000146
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Separation of organic–organic liquid mixtures using membranes has been investigated extensively over the past several decades due to its great significance in the chemical industry, Aromatic-alkane, alkane alkene and aromatics removal from gasoline systems among the most studied. These compounds are often present at very low concentrations in the mixtures and their recovery with traditional techniques, such as distillation and partial condensation, are not very simple. Pervaporation (PV) has been considered as one of the most active and promising areas in membrane technologies. In PV, the membrane itself is the key Polyvinyl chloride (PVC)-based mixed matrix membranes were prepared with LDH layered double hydroxide (NiFeCO3) particles, and were studied for the separation of toluene-heptane mixtures. It was found that the PVC transport properties could be significantly modified by the amount of NiFeCO3 incorporated. NiFeCO3/PVC membranes were characterized by IR FT spectroscopy and ATG.
Keywords: Composite membrane; Membrane separation; Pervaporation; Aromatic-aliphatic.
Abbreviations: GO: Graphene oxide; NaAlg: Sodium alginate; LHD: Layered double hydroxide; NaMMT: Sodium Montmorillonite; IPA: Isopropanol Nanocor; Organo-clay was prepared by ion exchange of NaMMT with Octadecylamine; Magh H: Maghnite-H; Algerian montmorllonite; PA: Polyamide; PDMS: Polydimethyl siloxane; PAN: Poly(Acrylonitrile); PEBA: Poly (ether-block-amide); PVA: Polyvinylalcohol; PVAc: Polyvinylacetal; PVC: Poly(vinylchloride); PPVC: Plasticized PVC; Rh B: 07, 7 wt% of Rh/H-b-zeolite; SDS: Sodium dodecyl sulfate; α-CD: α-Cyclodextrin; β-CD: β-Cyclodextrin.
Introduction
Separation of the aromatic-aliphatic fractions of industrial cuts, such as ethane-ethylene or benzene-cyclohexane, is an important goal in the petrochemical industry. Unfortunately, because the physical properties of the saturated and unsaturated compounds are similar, the conventional industrially used separation methods, i.e., adsorption, distillation, and liquid-liquid extraction, are not very efficient and can even be energy demanding because of the formation of azeotropes or the lack of significant volatility differences of the close boiling components [1,2]. Compared with these methods, pervaporation technique, as a new and efficient method, has advantages in both economy and environment. The main reason is that the separation performance of the pervaporation membrane cannot satisfy industrial requirements. Therefore, the major task at present for the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures is to improve the separation performance of the membrane [3].
Various materials including polymeric, inorganic and hybrid have been tried. Among them, polymers are primarily and the most extensively used PV membrane materials owing to their easy processing, satisfactory mechanical stability, controlled and tunable transport properties and low cost. However, the low chemical and thermal stability, poor resistant towards hazardous environment, and especially the inherent trade-off relation between permeability and selectivity limit the applicability of such polymeric membranes. On the other hand, a unique type of membrane, known as mixed matrix membrane (MMM), can be fabricated via employing two or more different materials of diversified physicochemical nature and such membranes possess a continuous phase, usually a polymer, embedded through a second dispersed phase [4]. In MMMs, appropriate selection of different phases is important and in many cases, addition of a small amount of dispersed phase may improve the physicochemical properties and separation proficiencies. Recently, nanotechnology has been considered as one of the highest potential areas for resolving he technical challenges coupled with the separation and purification technologies. The development of distinct nanostructured materials reformed the conventional perception of separation media, streaming new separation methods that outstrip the contemporary accomplishments with their unique properties.
The development of superior membrane materials in terms of both permeability and selectivity, fabricated from novel polymers and other NMs, and their commercialization into long-life stable modular configurations open the paths towards real time application of PV separations.
The aim of the present work is to develop a novel and high-performance mixed matrix membrane for the separation of organic molecules using PVC and LDH(NiFeCO3) particles were chosen for their higher affinity for toluene versus heptane. The hybrid membranes were characterized by TGA and IRFT.
Experimental
Reagents
The PVC, was purchased from ENIP of Skikda (ALGERIA), has an average molecular mass in weight of approximately 149.1 kg/mole. NiFeCO3 prepared by Boukraa et al. [5].
Preparation of pure and composite films
The thicknesses between (20.10-6 and 50.10-6 m) was performed from THF polymer solutions (12 wt%) by dispensing samples into 6 ×10-2 m diameter molds at a stirring speed of 280 tr/min. For composite films, particular attention was given to proper dispersion of the inorganic particles by pouring in the polymer matrix in order to ensure good contact with the polymer phase. Hence, a suspension of NiFeCO3 in 7 ml of THF were stirred for 24 h and was slowly added to the polymer solution and then stirred for 1 h and sonicated by batch ultrasonic, 1 h before casting into the glass molds. The membranes were allowed to dry slowly at room temperature, and hybrid films up to 40 wt% of NiFeCO3 were prepared. The pure PVC films were colorless, whereas the hybrid films were yellow and brown (Supplementary information).
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectral measurements were performed using a Nicolet spectrophotometer scanning from 400 to 4000 cm−1.
Thermo-gravimetric analysis (TGA)
The thermal stabilities of the membranes and NiFeCO3 were evaluated using the thermo gravimetric analyzer (TGA ) Mettler Toledo TGA/DSC1.
Sorption measurements
Sorption measurements of the PVC [6] and hybrid films was performed by immersion in pure liquids (toluene, n-heptane) and 50% wt toluene- heptane mixture at room temperature (25 ± 1°C). The samples, weighing at least 5 × 10-4 kg each, were submerged in the liquids until the sorption equilibrium was reached, approximately 24 h. Before each measurement, the samples were rapidly blotted, and the weight increase was recorded using a hermetically sealed flask. The measurements were repeated several times (relative error: ± 3%). The degree of swelling (Sw) was calculated for each sample using the following equation:

where Ww and Wd are the weights of the membrane in the wet and dry states, respectively.
Pervaporation measurements
These measurements were carried in continuous mode with a traditional equipment by maintaining constant the concentration upstream and a vacuum downstream lower than 100 Pa [7]; The PV experiments lasted several days to register the effect of feed composition were validated when the mean deviation between successive traps was below ± 5% for fluxes and ± 2% for selectivity (Supplementary information). The value of flux is calculated according to the formula: Eq 2.
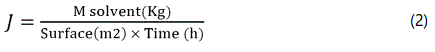
It is standardized for a 10 microns thickness in order to allow a direct comparison between the results obtained and films different thickness.
At the time of the study of mixtures, the composition of the permeate, determined by gas chromatography (FID), makes it possible to know enrichment. The Cp and Cf values are the weight fractions of either toluene or heptane in the permeate and feed, respectively. Noted α is selectivity of separation Eq 3; its maximum value towards + the infinite one. The Cp/Cf ratio is factor enrichment called β [8]; it should be noted that the 1/Cf possible maximum for a value β=1 indicate to a transfer without enrichment.


Results and Discussion
Membrane characterization
Fourier Transform Infrared Spectroscopy (FTIR): Infrared spectroscopy was used to obtain additional structural information and to characterize the functional groups present and the molecular interaction between PVC and NiFeCO3 with the membranes.
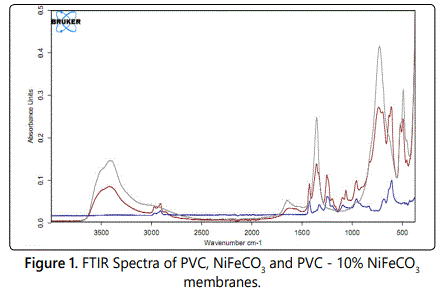
It showed a broad band between 4000 and 3000 cm−1 (Figure 1), three broad and intense bands are observed, which are attributed to the twisting vibrations of physisorbed water, vibrations of the structural OH− groups, characteristic valency vibrations of OH···OH, and/or characteristic stretching vibrations of M–OH in hydroxycarbonate.
Comparison of the spectra of the modified and unmodified materials reveals the appearance of a new band at 1080 cm−1 in the hybrid membranes designed C-O-C.
Thermal stability: thermo-gravimetric analysis (TGA)
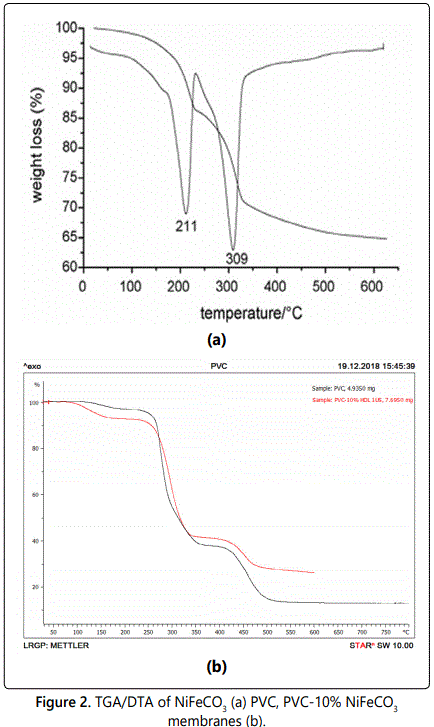
Figure 2a shows a DTA/TGA thermogram of the NiFeCO3 materials, two prominent endothermic peaks (at 210 and at 310°C) are seen in the DTA curve and they were both assigned to the elimination of water and CO2, respectively. Two weight loss stages were observed for NiFeCO3 in the TGA curve, coinciding with two endothermic peaks in the DTA profiles. The first weight loss at 210°C was ascribed to the loss of physically absorbed and interlayer water and the second weight loss in the temperature range from 240 to 600°C could be attributed to removal of CO2 from the inter-layer carbonate anions and water molecules from condensation of hydroxyl groups from the brucite-like layers [9,10], thus lead-to the destruction of hydrotalcite structure and the formation of mixed MII xMIIIy Ozoxides.
Two mass-loss steps can be observed in the PVC curve. The first stage of decomposition (up to 350°C) is due to dehydrochlorination, and the second step is the decomposition of polyenes.
The hybrid membranes showed a remarkable improvement in the thermal stability compared to pure PVC. Similar curves for PVC- 10% NiFeCO3 membranes are shown in figure 2b.
Thermal stability up to 550°C for PVC- 10% NiFeCO3 membranes compared to pure PVC up to530°C.
Sorption properties
The mass transfer of homogenous polymeric membranes in pervaporation can be described by the well-accepted sorption–diffusion model [11]. For a single component, the permeability P of a dense film can be expressed according to Eq. (5), where D is the diffusion coefficient of the permeant, and S is the sorption coefficient.
However, in the pervaporation (PV) process, the permeability is rarely constant and can vary significantly with the concentration or the activity of the species of the partially vaporized species (Table 1). When a given species has a good affinity for the polymer, the degree of sorption increases exponentially manner with the increase of the species activity and can be modeled by the Flory–Huggins equation [12].
Quantification of the sorption behavior characterizes the amplitude of the interactions between the small molecules and the PV film and makes it possible to predict the selectivity expected with a given binary mixture [13].
For mixed-matrix films, such as those prepared with NiFeCO3 and PVC, it should be emphasized that the two matrices do not interact with the small molecules in a similar manner, and thus the sorption properties are not governed by the same laws. For the polymer matrix, the absorption interactions occur within the entire mass of the organic matrix. For NiFeCO3, the absorption interactions are limited to specific fixed sites on the surface created by the inorganic matrix. One of the simplest models used to describe adsorption is the Langmuir model, which uses a perfectly planar surface and a single molecule adsorption per site [14].
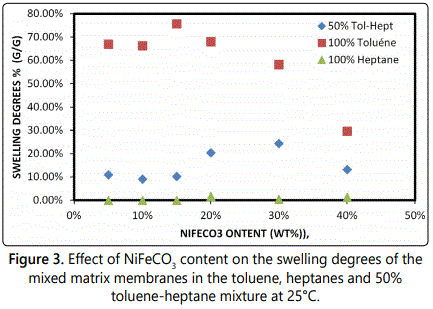
It was observed that NiFeCO3 had a strong affinity for toluene and exhibited a clear preferential sorption for toluene (1.3 times higher than that for heptane).
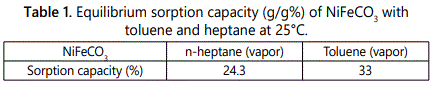
PVC–NiFeCO3 membranes: The sorption properties of the composite PVC–NiFeCO3 membranes were evaluated using toluene and n-heptane (Figure 3). Compared with the degree of swelling for unmodified PVC the composite membrane results show a marked increase in the solvent sorption that significantly varies with the NiFeCO3 content, as expected incorporation of NiFeCO3 into the PVC matrix increases the degree of swelling for both toluene and heptane. It can clearly be observed that the hybrid membranes have a much greater affinity for toluene.
Pervaporation
Separation by pervaporation requires the use of dense, non-porous polymer films. It is well recognized that the mass transfer is the result of the solution-diffusion mechanism that combines a thermodynamic step and a kinetic step [15]. Schematically, the performances are initially dependent on the affinities between the permeants and the polymer, which can be easily predicted from the sorption affinities and from the diffusion properties of the small molecules in the polymer matrix. Note that the sorption and diffusion phenomena can either favor or not favor the same molecule. Often, the selectivity registered with a binary mixture is significantly lower than the ideal selectivity that would be predicted from the pervaporation of each pure species; this is due to the coupling phenomenon between the two species, which is in any case, difficult to precisely analyze. Using rubbery polymers, the extent of coupling is typically the highest with organic-organic separation because the extent of polymer swelling can be very high.
Membrane performances
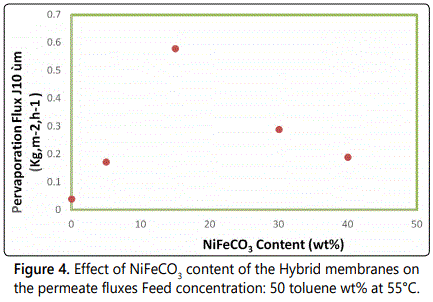
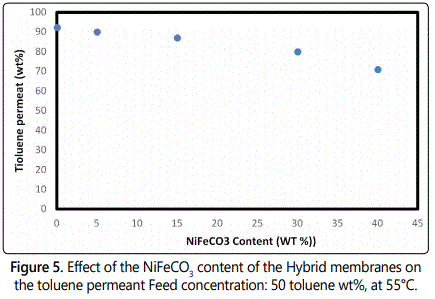
The properties of the Hybrid films were determined with the 50/50 toluene/n-heptane feed mixture at temperatures, 54°C [16,17]. The effect of incorporation of the NiFeCO3 on the selectivity and the PV flux are presented in figures 4 and 5.
For the pure PVC films, a strong toluene enrichment of the permeate is also obtained with all of the mixed matrix membranes, corresponding to a toluene percentage increase from 50 wt% in the feed up to 92 wt% in the permeate. Clearly, the incorporation of the NiFeCO3 filler does not introduce significant defects. The flux rises with increasing NiFeCO3 content, whereas the separation factor decreases with addition of NiFeCO3 [18,19].
Comparison with literature data
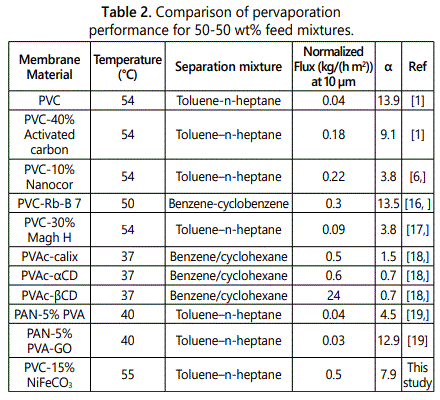
Many types of membranes have been used to study pervaporation separation of toluene-n heptane mixtures (Table 2) in aromatic-aliphatic separation.
The pervaporation performances of the as prepared PVC-15% NiFeCO3 membranes for aromatic/aliphatic mixtures were compared with the reported membranes as shown in table 2. The permeation fluxes for PVC-15% NiFeCO3 has higher than other membranes except PVAC-βCD but the factor separation α was better than PVAC-βCD membrane, this results were obtained for PVC-15% NiFeCO3.
Conclusions
These results show that a simple and economical polymer, such as PVC, can be used with an appropriate filler to obtain high separation properties for aromatic-alkane mixtures by pervaporation due to the intrinsic PVC affinity for aromatics induced by the polar chlorine atoms of the polymer backbone. To progress towards the potential applications of PVC membranes, thin, supported, dense selective layers should be prepared to drastically increase the permeance while maintaining constant the high selectivity due to the PVC matrix.
Acknowledgements
The authors sincerely thank E.Girot for recording analysis and USTO.M. Boudiaf University is also acknowledged for its financial support.
References