Research Article
Effects of Secondary reaction of Primary Volatiles on Oil/gas Yield and Quality from Oil Shale Pyrolysis
1State Key Laboratory of Heavy Oil Processing, China University of Petroleum-Beijing, Beijing 102249, P.R.China
2College of Materials Science and Engineering, Nanjing Forestry University, Nanjing 210037, Jiangsu, P.R. China
*Corresponding author: Yuming Zhang, State Key Laboratory of Heavy Oil
Processing, China University of Petroleum-Beijing, Beijing 102249, P.R.China, Tel: +86-15001296129, E-mail: ymzhcup@163.com
*Second Corresponding author:
Shu Zhang, College of Materials Science and
Engineering, Nanjing Forestry University, Nanjing 210037, Jiangsu, P.R. China, E-mail: s.zhang@njfu.edu.cn
Received: June 27, 2018 Accepted: July 7, 2018 Published: July 12, 2018
Citation: Zhang Y, Linghu R, Huang L, Zhang L, Sun G, Zhang S. Effects of Secondary reaction of Primary Volatiles on Oil/gas Yield and Quality from Oil Shale Pyrolysis. Int J Petrochem Res. 2018; 2(2): 155-161. doi: 10.18689/ijpr-1000127
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The reaction behaviors of primary volatiles from oil shale pyrolysis were investigated using a two-stage reactor under different in-situ thermal reaction conditions. The secondary reaction parameters, such as conversion temperature, reaction atmosphere and residence time, were studied for their effects on the pyrolysis oil/gas yield and quality. The results showed that the reaction temperature had a profound influence on the oil and gas yield, as indicated by the fact that the pyrolyzed shale oil and gas yield reduced by 15% (mass) and 20% (mass) respectively, with the temperature of 2nd stage reactor increasing from 600°C to 650°C. Comparing with a nitrogen atmosphere, the liquid oil yield could enhance by 5% when steam was added as the reaction atmosphere in the second stage, and the corresponding oil was mainly concentrated in the gasoline and diesel distillations, that is, boiling point <350°C. The GC-MS analysis illustrated that the secondary reaction was mainly pyrolysis at the residence time of 0~3 seconds while the presence of steam could increase the content of aliphatic hydrocarbons in the oil and meanwhile suppress the condensation of aromatics. When the residence time greater than 3~5 seconds, the secondary reaction was mainly condensation of poly-aromatics, which led to the increase of coke. Meanwhile, the gasoline and diesel oils yield remained stable, and the VGO fractions decreased by about 30%.
Key words: Oil shale; Pyrolysis volatiles; Steam reforming; Secondary reaction
Introduction
With the rapid development of society, the increasing demand for energy and the gradual decrease of fossil energy have not changed. According to BP Energy Statistics, China's oil consumption reached 560 million tons in 2015, while China's crude oil production was only about 210 million tons, and thus China's crude oil dependence has exceeded 60% [1]. In recent years, many scientific researchers have done a lot of research on renewable energy such as bioenergy [2] and solar energy, and oil shale has also been exploited to ease the pressure on traditional oil/gas. Among them, oil shale, as an alternative energy source for traditional petroleum, has abundant reserves in China. It conservatively estimates that a total of 400 billion tons of oil shale have been exploited, about one-tenth of the total amount in China. Oil shale resources are mainly distributed in 20 provinces and autonomous regions, 47 basins, a total of 80 mining area [3]. Therefore, how to transform and utilize oil shale resources efficiently is crucial to supplement the shortage of traditional oil and gas resources and guarantee the safety of China's energy structure [4].
Pyrolysis oil produced by low-temperature oil shale pyrolysis is an effective way to utilize oil shale resources, and improving the oil shale pyrolysis oil (hereinafter referred to as the pyrolysis oil) yield and the quality is of core importance for the development of pyrolysis technology. Experimental research and industrial application have found that just pursuing pyrolysis oil yield will often lead to the reduction of its quality and bring more difficulties to subsequent treatment. In the study of coal pyrolysis, Zhou et al [5] assume that the secondary reaction of a volatile can cause the heavy components to condense in a pyrolysis reactor, thus delaying and even reducing tar coking problem in subsequent processing. Therefore, using the secondary reaction in the reactor to regulate the primary volatiles generated by pyrolysis, and increasing the pyrolysis oil quality at the expense of a small amount of pyrolysis oil yield has attracted wide attention from researchers. A common research method is to use a two-stage reaction device, via placing the pyrolysis raw material in the first stage, and adding an inert/catalyst bed in the second stage to increase the pyrolysis product's pyrolysis residence time or further utilizing the catalysis materials in the second stage to achieve the secondary conversion of oil and gas. Hayashi et al. [6] showed that the use of non-catalytic quartz sand as bed material would allow a single thermal cracking of the primary pyrolysis product, which could improve oil and gas yield and composition. Lai et al. [7] used shale ash as the secondary load to study the pyrolysis reaction of Yilan oil shale, finding that the controlled residence time was 0~10 seconds and the pyrolysis oil yield was reduced by 30%. Meanwhile, the gasoline and diesel components in the pyrolysis oil have increased by 45%, and the coke produced in the second stage will cover the shale ash surface and reduce its catalytic activity. Espitalie et al. [8] demonstrated that if the heavy hydrocarbon products produced by the pyrolysis of kerogen remained on the surface of the minerals, these discharged heavy hydrocarbons would undergo secondary cracking on the surface of the minerals, and the residues finally converted into coke. However, in the inert atmosphere environment alone, the thermal cracking or catalytic cracking conversion method causes the second stage to accumulate a large amount of coke due to the aromatic condensation reaction. Song et al. [9] studied biomass pyrolysis and discovered that the steam generated by the in-situ biomass thermal conversion would cause more free radicals to adsorb with macromolecular tar and leading to reforming reaction. The addition of steam can effectively inhibit the polymerization of aromatics to reduce the hard decomposition components of tar, and increase the production of aliphatic hydrocarbons so that the subsequent tar cracking or reforming process becomes easy [10, 11].
The production of oil and gas from oil shale pyrolysis in the industry also faces the problems of heavy components of liquid oil products and difficulties in subsequent processing and utilization. At the same time, in the commonly used reactors, such as moving beds and rotary furnaces, the primary volatile products generated from pyrolysis are inevitably affected by the internal temperature within the reactor, the reaction atmosphere and the residence time, and simultaneously the secondary reactions occur. However, in the study of oil shale pyrolysis, it is rarely reported the secondary reactions, such as steam atmosphere, reaction temperature and cracking time. Also, the secondary transformation of primary products of oil shale pyrolysis and their corresponding reaction mechanisms need to be studied. In this study, steam was used as a secondary conversion additive. First, inert quartz sand was used as the secondary bed material (oil shale ash and catalysts are used in the following study.) in the non-catalytic environment. The effect of the steam in the non-catalytic environment at different temperatures and residence times are investigated on the secondary thermal reaction conversion. The characteristics of pyrolysis oils were further studied in combination with simulated distillation and GC-MS characterization methods, which led to a basic analysis of the secondary transformation of primary pyrolysis products of oil shale and coke formation and to guide the development and design of oil shale pyrolysis processes.
Experimental
Materials
The experimental raw material used in this paper was
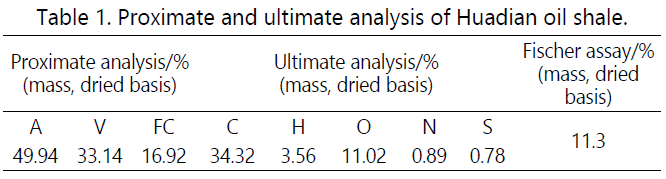
Huadian oil shale. The results of the proximate analysis, ultimate analysis, and Fischer assay are shown in Table 1. Before the experiment, the oil shale was first crushed and
sieved to particles with a particle size of 1~1.8 mm, and then
placed in a constant temperature blast drying oven and dried
at a constant temperature of 120° C for 5 hours. The particle
size of quartz sand used in the second stage during the
experiment was 0.45~1 mm. It was calcined in a muffle furnace
at 800°C for 1 hour in advance and then dried at a constant
temperature for 3 hours. Table 1 shows that the ash content of
Huadian oil shale sample is 49.94% with the volatile content to
be 33.14%, and the oil yield of Fisher assay is about 11.3%.
Apparatus
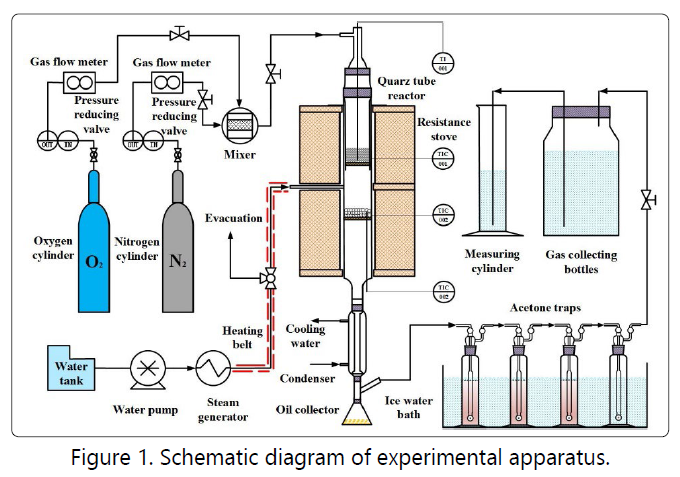
The experimental device used in this paper is shown in
Figure 1. The main part is a two-stage quartz reactor, in which
the upper section is used for rapid pyrolysis of oil shale and
the lower section is for secondary conversion of pyrolysis
products. The second section of the reactor is provided with a
bypass pipe for the addition of steam or other reaction
atmospheres. Both the upper and lower part of the reactor is
equipped with a temperature controller for monitoring and
controlling the temperature. High-purity nitrogen is controlled
by the mass flow meter as a purge gas and enters the reactor
at different gas rates from the top.
Before the start of each experiment, a certain amount of quartz sand was placed on the lower reactor distribution plate. After the device was connected, the upper and lower parts of the reactor were separately heated and reached the required temperature with the carrier gas purging the whole system. 20 g of oil shale was rapidly added from the top cover, thus to achieve rapid pyrolysis of oil shale. High-temperature steam with certain flow rates at 350°C enters from the middle of the two-stage reactor. The volatiles from the upper stage and the hot steam reacted with each other in the lower stage and also contacting with the loaded quartz sand. Then the reaction product was rapidly cooled in the condenser. The uncondensed oil and gas products entered three acetone bottles in the ice water bath so that the oil could be fully absorbed. The remaining oil and gas were then trapped by a high-efficiency filter. The mass of the filter was weighed before and after the reaction to confirm that the oil was completely condensed or absorbed by the acetone. Noncondensing pyrolysis gas finally collected in the determined volume by the drainage and gas collection method and using gas chromatography to detect the pyrolysis gas composition.
Product characterization and analysis
Simulated distillation (Agilent 7890) are used to determine
the composition of pyrolysis oil with different boiling fractions. In this study, pyrolysis oil is divided into three major
components according to its boiling point, that is, gas-diesel
distillate (boiling point <350 °C), vacuum gas oil (boiling point
at 350-500 °C), heavy distillate oil (boiling point >500 °C). The
pyrolysis oil was also analyzed for its material and elemental
composition using a gas chromatography coupled online
with a mass spectrometer (GC-MS, Agilent 7890A). Noncondensable
gas was analyzed by micro gas chromatography (INFICON Micro3000) to obtain the contents of hydrogen, methane, carbon monoxide, carbon dioxide, ethylene, ethane, propylene, and propane. Gas components such as ethylene, ethane, propylene, and propane were uniformly labeled as
C2~C3 hydrocarbon components for easy analysis.
During the two-stage pyrolysis experiment, the volatiles from the first stage was rapidly entrained out of the hightemperature reaction zone and the residence time in the second stage bed was adjusted by adjusting the height of the second quartz sand bed and the gas flow rate. This study mainly investigated the effect of the second stage reaction conditions of the reactor on the primary volatile components. In the pre-experiments, the conditions for maximizing the liquid yield of the first section of oil shale pyrolysis were determined, and then the reaction conditions such as the first pyrolysis temperature and residence time were fixed. As a result, the pyrolysis oil and gas yield and quality were optimized by adjusting the different atmospheres (nitrogen or steam) and residence time of the second reactor.
Results and Discussion
Overall variation of oil and gas
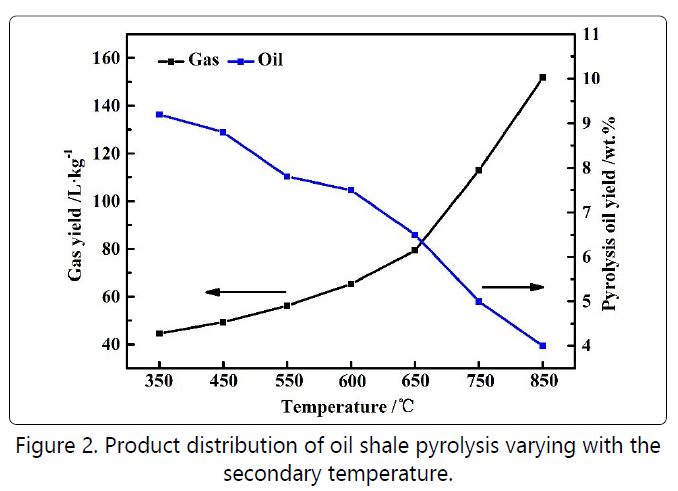
Figure 2 shows the change in oil and gas production and
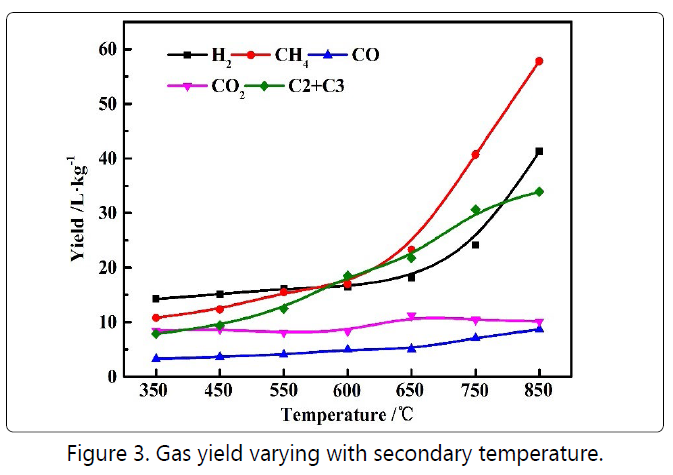
Figure 3 illustrates the gas composition as a function of the
reaction temperature in the second stage. With the
temperature reaching 600°C and 650°C, the yield of the oil
was greatly reduced. Meanwhile, the gas yield such as
hydrogen, methane, and C2+C3 increased significantly, indicating that the temperature of 600°C might be the critical
temperature for the strengthening of the secondary reaction. When the temperature continued rising to 750°C and 850°C, the secondary conversion was so severe that the oil yield
dropped sharply with greatly increased the gas production. Therefore, based on the idea of losing minimum oil yield and
improving oil and gas quality, 600°C and 650°C were selected
as the inspection temperatures during the regulation of the
two-stage reaction condition.
Previous experiments have shown that when the upper stage temperature was 600°C in the nitrogen atmosphere, the reaction time of 25 minutes was necessary to ensure the completion of oil shale reaction with the maximum pyrolysis oil yield to be 9.5% by mass. Therefore, the first stage of pyrolysis in the following two-stage analysis was based on the above conditions. By adding steam and changing the second stage temperature, the conditions for optimizing oil, gas yield and quality were comprehensively investigated. Figure 4 shows that the yield of oil shale pyrolysis oil continued to decrease at longer residence time. S-600 (Steam) and N-600 (Nitrogen) represented adding steam and nitrogen in the second stage, respectively, with the reaction temperature being 600°C.From the overall trend of the curve, the oil yield of the second stage via adding steam was higher than that of nitrogen, and the effect of the reaction temperature on the oil yield was much greater than the effect of the steam and the residence time. Thus, the oil yield presented the ordering pattern of S-600>N-600>S-650>N-650.
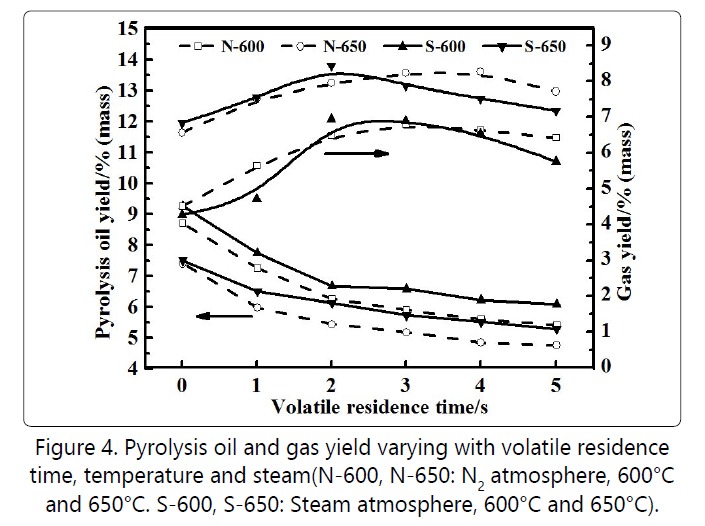
In general, the secondary cracking of volatiles had the most obvious effect on the change of oil and gas [12]. By increasing the height of quartz sand and changing the residence time from 0 to 1 second, the yield of pyrolysis oil was greatly reduced, while the gas significantly increased due to the rapid cracking of oil components. With the residence time being 1~3 seconds, the yield of pyrolysis oil dropped rapidly because the addition of quartz sand increased the mass transfer resistance of the gaseous products. Williams et al. [13] found that the secondary conversion of primary pyrolysis components was greatly influenced by temperature and diffusion, which ultimately led to differences in the reaction process and products. When the residence time was 3~5 seconds, the rate of decrease of pyrolysis oil yield became slow due to the weaker secondary reaction. The above changes in the pyrolysis of oil and gas were mainly caused by the following two reasons. First, as the residence time increased in the first period (<3 seconds), the macromolecular cracking generated more gas molecules because of the secondary pyrolysis. With the increase of residence time, the pyrolysis reaction was saturated and slower. Second, the resistance to the diffusion of a large amount of gas generated by rapid cracking increased, thereby enriching the secondstage reaction space. As a result, it may inhibit the reaction from increasing toward the larger volume of the product. In the absence of steam, the consumption of secondary pyrolysis resulted in a reduction of about10-15%in oil yield. Steam as an additive may have a certain inhibitory effect on the secondary conversion of primary pyrolysis products of oil shale.
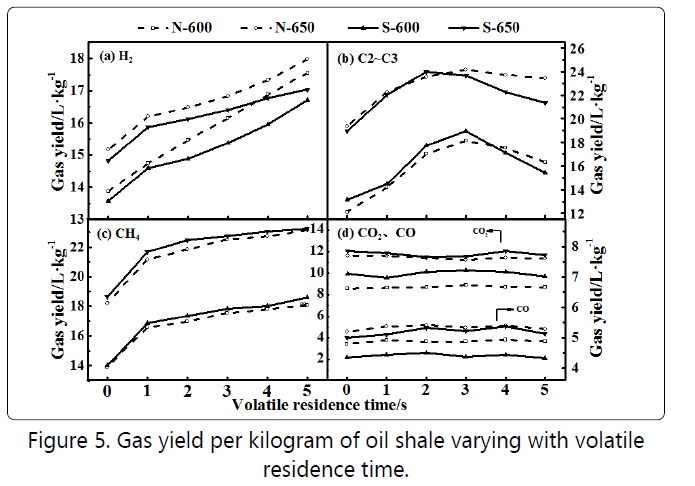
Figure 5 shows the composition of the pyrolysis gas or oil shale with unit mass as a function of the residence time in the second stage. The production of the hydrogen in Figure 5(a) and the methane production in Figure 5(c) increased with increasing residence time, while the production of C2-C3 in Figure 5(b) increased first and then decreased. As the residence time increases, the condensation process intensifies, and the hydrogen production also increases accordingly. Wiktorsson et al. reported that hydrogen was mainly from cyclization, aromatization, and condensation reactions of aromatics [14]. There was a decrease in the overall hydrogen yield via adding steam, indicating that steam had an inhibitory effect on the condensation of aromatic hydrocarbons. Methane was produced by the cleavage and hydrogenation reaction of the methyl side chain [15]. After adding steam, the methane production increased slightly, which might be caused by the reaction of steam with macromolecules in the volatiles.C2~C3 mainly comes from the thermal cracking process of heavy aliphatic hydrocarbons [16], so its change trend also complied with the fact that the thermal cracking process first increased and then weakened. Figure 5(d) justified that carbon dioxide and carbon monoxide mainly came from the cleavage of carboxyl and carbonyl groups [17] because there was no significant change as the residence time increases.
Oil distillation range analysis
In order to thoroughly explore the mechanism and process
of secondary transformation, the experimental groups with
retention times of 0 second, 1 second, 3 seconds and 5 seconds
at a reaction temperature of 600°C were selected to study the
effect of steam on the structural composition of pyrolysis oil
fractions. It was found that due to the reaction of pyrolysis
products of oil shale in the second stage, the heavy components
with a temperature greater than 500°C mostly changed into
coke. Therefore, heavy components only existed at a residence
time of 0 seconds (without silica sand), and no heavy oil
components were formed at other residence times. The
secondary cracking of the volatiles could convert the heavy oil
components in the liquid and the resulting light oil was easier to
post-process. Figure 6(a) shows the relative composition of the
components of the pyrolysis oil for each residence time. The
relative content of the gasoline-diesel fraction exceeded 60% regardless of the addition of steam. Regardless of the residence time, the content of light oil components after adding steam
was higher than that of without steam, which was relatively
increased by 4% to 5%.This further shows that the reforming
effect of steam was beneficial for increasing the yield of light oil.
Figure 6(b) shows the absolute content of each fraction of the pyrolysis oil. When the residence time was 0~1 second, due to the increase of the second-stage quartz sand bed material, the oil composition was abruptly changed, and the secondary conversion of the heavy oil component resulted in a significant increase in the content of the light oil component [18]. The light oil and VGO components in Figure 6(b) decrease slowly with increasing residence time within 1~3 seconds. The cracking of the heavy oil component increased at larger residence time, but at the same time there was a decomposition of the light oil component and resulted in a significant increase in the gas yield. The secondary conversion in this process is mainly based on decomposition reactions [19].
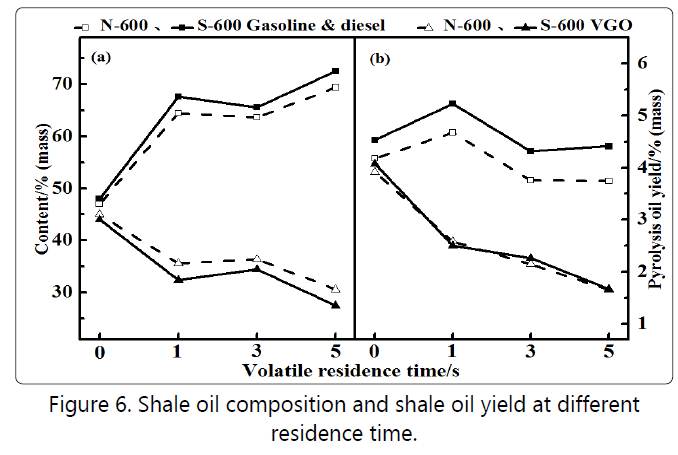
The second conversion of a large number of enriched gases inhibited the continued generation of gas within 3~5seconds. The cracking process of the light oil components tended to be saturated, which was consistent with the rapid increase of hydrogen and methane in the gas composition of Figure 5, indicating that this stage was mainly the condensation process of heavy oil fractions of pyrolysis oil [20]. In addition, comparing the analysis of oil yield and hydrocarbon composition in Section 3.1, it can be seen that as the residence time increased, its effect on the secondary conversion of the primary product gradually weakened. Comparing the light and heavy component yields of pyrolysis oil, the effects of steam addition were prominent only at the initial stage. The regulation effect of steam on the secondary conversion of the primary product of oil shale pyrolysis could not only reflect the yield of pyrolysis oil but also play a key role in the conversion of light and heavy components, which was of great benefit to improve the quality of pyrolysis oil.
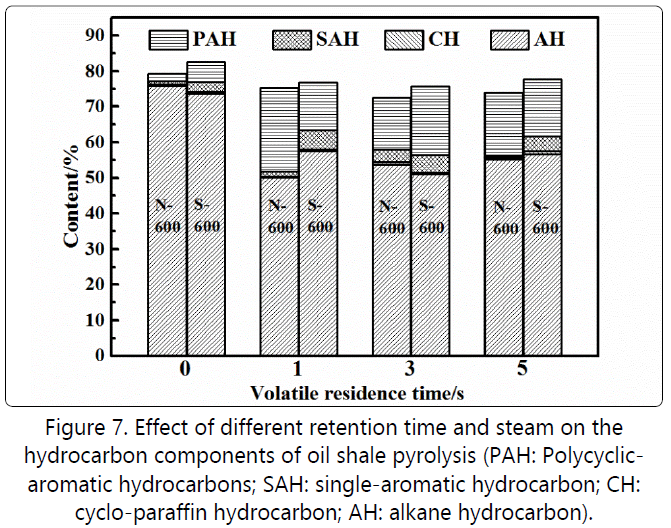
Changes in oil composition Figure 7 further analyzes pyrolysis oil composition by using gas chromatography-mass spectrometry (GC-MS). Here, PAH represents polycyclic aromatic hydrocarbons, SAH being single aromatic hydrocarbon, CH being Cyclo-paraffin hydrocarbon and AH means Alkane hydrocarbon. The hydrocarbon components in pyrolysis oil all exceeded 70%, with the content of aliphatic hydrocarbons >50%, and the content of aromatic hydrocarbons being 20%. After adding steam, the content of hydrocarbons was slightly higher than that without steam. After the addition of steam, the content of aromatics increased because steam might relieve the condensation reaction of aromatic hydrocarbons, and some non-hydrocarbons undergo steam cracking reactions [21]. Increasing the silica sand bed also caused the inhibitory effect of steam on the polymerization of aromatics obviously. Therefore, the proportion of monocyclic aromatics (20%) via adding steam was much higher than that without steam (2%). The proportion of aliphatic hydrocarbons in the residence time from 1 second to 3 seconds increased by 5%in the absence of steam, while the total aromatics content also decreased by nearly 7.5%. The molar ratio of hydrogen to carbon increased from 1.74 to 1.85.
Combined with the analysis of the pyrolysis oil distillation range and gas evolution rule in Figure 6, it can be seen that the increase in residence time led to the increased cracking of aliphatic hydrocarbons, resulting in an increase in volume fraction. When the residence time was 3~5 seconds, the secondary cracking became weak, and the condensation process intensified. Figure 5 shows that the production of C2~C4 in the gas decreased and the hydrogen increased slowly. This shows that the secondary pyrolysis had obviously changed from the condition of equal weight between cracking and condensation to that of condensation [22].
When steam was added, the molar ratio of hydrogen to carbon in the pyrolysis oil was reduced from 1.86 to 1.79 during the residence time of 1 to 3 seconds. The aromatic content increased by 5.4%, with the content of aliphatic hydrocarbons reducing by 6.5%. It can be seen from Figure 6 that the content of light components was still higher than that without steam, justifying that the addition of steam could well suppress the condensation of aromatics. The coking process was effectively controlled, but the aromatic components in the pyrolysis oil increased making the oil to be lighter. In addition, the addition of steam promoted cracking of aliphatic hydrocarbons, corresponding to a significant increase in the gas yield during the 1~3 seconds residence time period in Figure 4 [23]. However, the condensation process intensifies and the steam became weaker at longer residence time and the aromatic content reduced by 4%.[24][25].
Conclusion
In this study, two stages of pyrolysis reaction devices were used to comprehensively examine the effects of secondary conditions such as steam addition, temperature and residence time on the primary pyrolysis products of oil shale. The steam was used as an additive for secondary conversion, and quartz sand was used as a two-stage bed material to study the effect of steam at different temperatures and residence times on the secondary conversion of pyrolysis oil and gas products. Based on the requirements of minimum reduction of oil and gas yields, the operating parameters and the corresponding reaction routes for improving the quality of pyrolysis oil and gas products were obtained, and the following conclusions were obtained.
(1). The reaction temperature has the most obvious effect on the oil and gas yield. With the second stage temperature increasing from 600°C to 650°C, the pyrolysis oil yield was reduced by 15%, and the gas yield was increased by about 20%. Compared with the nitrogen atmosphere, the addition of steam in the second stage as the reaction atmosphere increased the liquid oil yield by about 5%, and the pyrolysis oil was mainly concentrated in the gasoline and diesel fractions(boiling point <350°C). The long residence time at high temperature caused the oil over-cracking to generate gas and coke.
(2). The height of quartz sand bed was used as a parameter to control the residence time. With the increase of the residence time, the influence on the secondary pyrolysis of primary volatiles gradually weakened. The heavy components were converted into light components via contacting with silica sand. Within 1~3 seconds of the residence time, the cracking of aliphatic hydrocarbons intensified, while the gas yield increased significantly and mainly dominated by decomposition reactions. During the 3 to 5 seconds residence time, the yield of VGO components was significantly reduced. The rapid enrichment of gas and the presence of steam could suppress gas generation and this process mainly dominated by the condensation reactions.
(3). The addition of steam had an inhibitory effect on the polymerization of aromatics. The proportion of SAH monocyclic aromatics in the aromatic component after the addition of 20% steam was much higher than that without steam. This shows that the addition of steam could well inhibit the condensation of aromatics. In general, the regulation effect of steam on the secondary reactions of oil shale pyrolysis volatiles was not only reflected in the increase in oil yield but also could improve the content and quality of light components in oil products, which reduced the difficulty in the downstream processing of oil products.
Acknowledgments
The study was conducted with the research programs financed by National Natural Science Foundation (21406264), National Basic Research Program of China (2014CB744304) and Science Foundation of China University of Petroleum, Beijing (No. 2462018BJC003).
References
- British Petroleum. Statistical review of world energy 2016, UK, Pureprint Group, 2016.
- Atashbar NZ, Labadie N, Prins C. Modelling and optimization of biomass supply chains: a review. International Journal of Production Research. 2018; 56(10): 3482-3506. doi: 10.1016/j.ifacol.2016.07.742
- Atashbar NZ, Labadie N, Prins C. Modelling and optimization of biomass supply chains: a review. International Journal of Production Research. 2018; 56(10): 3482-3506. doi: 10.1016/j.ifacol.2016.07.742
- Yan F, Song Y. Properties Estimation of Main Oil Shale in China. Energy Sources, Part A. 2009; 31(4): 372-376. doi: 10.1080/15567030701530347
- Zhou Q, Liu Q, Shi L, Yan Y, Liu Z. Behaviors of coking and radicals during reaction of volatiles generated from fixed-bed pyrolysis of a lignite and a subbituminous coal. Fuel Processing Technology. 2017; 161: 304-310. doi: 10.1016/j.fuproc.2017.01.040
- Hayashi JI, Iwatsuki M, Morishita K, Tsushi A, Li C, Chiba T. Roles of inherent metallic species in secondary reactions of tar and char during rapid pyrolysis of brown coals in a drop-tube reactor. Fuel. 2002; 81(15): 1977-1987. doi: 10.1016/S0016-2361(02)00128-X
- Lai D, Chen Z, Lin L, Xu G. Secondary cracking and upgrading of shale oil from pyrolyzing oil shale over shale ash. Energy & Fuels. 2015; 29(4): 2219- 2226. doi: 10.1021/ef502821c
- Espitalie J, Makadi KS, Trichet J. Role of the mineral matrix during kerogen pyrolysis. Organic Geochemistry. 1980; 64(1): 59-66.
- Song Y, Wang Y, Hu X, Xiang J, Hu S, Mourant D, et al. Effects of volatile– char interactions on in-situ destruction of nascent tar during the pyrolysis and gasification of biomass. Part I. Roles of nascent char. Fuel. 2014; 122(122): 60-66. doi: 10.1016/j.fuel.2014.01.002
- Minkova V, Razvigorova M, Bjornbom E, Zanzi R, Budinova T, Petrov N. Effect of water vapour and biomass nature on the yield and quality of the pyrolysis products from biomass. Fuel Processing Technology. 2001; 70(1): 53-61. doi: 10.1016/S0378-3820(00)00153-3
- Sou H, Kumabe K, Ohshita M, Norinaga K, Li C, Hayashi J. Mechanism of decomposition of aromatics over charcoal and necessary condition for maintaining its activity. Fuel. 2008; 87(13-14): 2914-2922. doi: 10.1016/j. fuel.2008.04.019
- Chen Y, He R. Fragmentation and diffusion model for coal pyrolysis. Journal of Analytical and Applied Pyrolysis. 2011; 90(1): 72-79. doi: 10.1016/j.jaap.2010.10.007
- Williams PT, Ahmad N. Influence of process conditions on the pyrolysis of Pakistani oil shales. Fuel. 1999; 78(6): 653-662. doi: 10.1016/S0016- 2361(98)00190-2
- Wiktorsson LP, Wanzl W. Kinetic parameters for coal pyrolysis at low and high heating rates-a comparison of data from different laboratory equipment. Fuel. 2000; 79(6): 701-716. doi: 10.1016/S0016-2361(99)00138-6
- Xu WC, Tomita A. Effect of temperature on the flash pyrolysis of various coals. Fuel. 1987; 66(5): 632-636. doi: 10.1016/0016-2361(87)90271-7
- Nazzal JM. The influence of grain size on the products yield and shale oil composition from the pyrolysis of Sultani oil shale. Energy Conversion and Magagement. 2008; 43(6): 663-668.
- Xiong R, Dong L, Yu J, Zhang X, Jin L, Xu G. Fundamentals of coal topping gasification: characterization of pyrolysis topping in a fluidized bed reactor. Fuel Processing Technology. 2010; 91(8): 810-817. doi: 10.1016/j. fuproc.2009.07.005
- Sonoyama N, Nobuta K, Kimura T, Hosokai S, Hayashi J, Tago T, et al. Production of chemicals by cracking pyrolytic tar from Loy Yang coal over iron oxide catalysts in a steam atmosphere. Fuel Processing Technology. 2011; 92(4): 771-775. doi: 10.1016/j.fuproc.2010.09.036
- Zhang J, Kong LW, Bai JR, Wang Q. Pyrolysis Characteristics of Oil Shale of Six Density Sections. Energy Procedia. 2012; 17: 196-201. doi: 10.1016/j. egypro.2012.02.083
- Jameel S, Alhesan A, Fei Y, Marshall M, Jackson WR, Qi Y, et al. Long time, low temperature pyrolysis of El-Lajjun oil shale. Journal of Analytical and Applied Pyrolysis. 2018; 130: 135-141. doi: 10.1016/j.jaap.2018.01.017
- Parthasarathy P, Narayaman KS. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield-A review. Renewable Energy. 2014; 66: 570-579. doi: 10.1016/j. renene.2013.12.025
- Li Q, Han X, Liu Q, Jiang X. Thermal decomposition of Huadian oil shale. Part 1. Critical organic intermediates. Fuel. 2014; 121: 109-116. doi: 10.1016/j.fuel.2013.12.046
- Salaices E, Serrano B, Lasa HD. Biomass catalytic steam gasification thermodynamics analysis and reaction experiments in a CREC riser simulator. Industrial & Engineering Chemistry Research. 2010; 49(15): 6834-6844. doi: 10.1021/ie901710n
- Chen B, Han X, Li Q, Jiang X. Study of the thermal conversions of organic carbon of Huadian oil shale during pyrolysis. Energy Conversion and Management. 2016; 127: 284-292. doi: 10.1016/j.enconman.2016.09.019
- Lai D, Shi Y, Geng S, Chen Z, Gao S, Zhan JH, et al. Secondary reactions in oil shale pyrolysis by solid heat carrier in a moving bed with internals. Fuel. 2016; 173: 138-145. doi: 10.1016/j.fuel.2016.01.052



