Research Article
Effects of lean alkanolamine temperature on the performance of CO2 absorption processes using alkanolamine solutions
Chemical engineering department, Isfahan University of Technology, Isfahan, Iran
*Corresponding author: Abolghasem Kazemi, Chemical engineering department, Isfahan University of Technology, Isfahan, Iran, Tel: +989171492783, E-mail: abolghasemkazemi@gmail.com
Received: May 26, 2018 Accepted: May 30, 2018 Published: June 4, 2018
Citation: Kazemi A. Effects of lean alkanolamine temperature on the performance of CO2 absorption processes using alkanolamine solutions. Int J Petrochem Res. 2018; 2(1): 141-147. doi: 10.18689/ijpr-1000124
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Acid gas removal from the natural gas using alkanolamine processes is the most common technology used for sweetening of natural gas. Based on the sour and sweet gas specifications, several alkanolamine solutions can be used for acid gas removal, all of which are well developed processes. However, one of the remaining issues is the costs associated with the processes. In this study, DEA, DGA and mixed (MDEA+DEA) processes are designed for sweetening the natural gas produced in one of the gas fields having high CO2/H2S ratio. For each process, seven scenarios are designed to investigate the effects of the coolerʼs operating parameters on the performance of the process. For each scenario, the duty of the cooler is varied in order to have a specific lean amine temperature entering the absorber. Each scenario is simulated using Aspen HYSYS and economically evaluated using Aspen economic evaluation. Based on the results of this study, the required solution circulation rates slightly increases when the lean amine temperature increases. However, Lower process capital costs and lower coolerʼs duty were obtained by operating the DEA and DGA processes at higher values of lean amine temperature. Also, operating at lower lean amine temperatures resulted in lower hydrocarbon pick up in case of MDEA+DEA process.
Keywords: CO2; Natural Gas Sweetening; Coolerʼs Parameters; DEA; DGA; Mixed Amine
Introduction
The processes using Alkanolamine solutions for acid gas removal from natural gas are the most common processes used for the removal of acid gases from natural gas. The alkanolamine processes are well developed processes, each of which is suitable for sweetening the natural gas with certain sour and sweet gas specifications [1-9]. However, one of the main issues is the large costs associated with these processes [10-12]. Numerous studies have been carried out to reduce the costs associated with these processes.
Polasek et al studied alternative flow schemes for natural gas sweetening [11], Bae et al studied split flow configuration for the process [13], Warudkar et al studied the effects of stripper operating parameters [10], Cousins et al studied modifications on the process flow sheet [14], Sohbi et al and Fouad et al studied effects of using mixed alakanolamines [6, 7], Kazemi et al and Ghanbarabadi et al performed comparative studies between different processes [15, 16], Nuchitprasitichai et al, Øi et aland Mores et al used optimization techniques [12, 17, 18] Freeman et al proposed using concentrated piperazine mixtures [8] and Banat et al used energy analysis method [19] for reducing costs and energy requirements of the sweetening processes.
For the sweetening of the natural gas with certain specifications, several processes might be applicable. One of the questions which arise in these situations is that which process is the most economical process to be used for sweetening of the natural gas with these specifications? Also, one of important parameters which affect the costs associated with a sweetening process is the lean solution temperature entering the absorber. Changing the lean amine temperature might have an impact on the solution flow rate needed in order to reach the wanted specifications of sweet natural gas which strongly affects the costs associated with the natural gas sweetening processes. On the other hand, the choice of lean amine temperature, affects the duty that is needed to be applied on the cooler. Another question is that at what temperature, should the lean solution enter the absorber in order to have the best economic performance? In this study I tried to answer these two questions for the case of the natural gas produced in a gas field having high CO2 /H2S ratio, which has relatively high CO2 and low H2S contents and has low pressure.
In this study, the effects of coolerʼs operating parameters are investigated. The DEA, DGA and mixed(MDEA+DEA) processes are designed for sweetening of the natural gas produced in a gas field having high CO2 /H2S ratio. Seven scenarios are designed to investigate the effects of coolerʼs operating parameters on the performance of the processes. Each scenario is simulated using Aspen HYSYS and economically evaluated using Aspen economic evaluation. The results of simulation and economic evaluation are then studied to select the optimum operating conditions for the processʼs cooler. Although there have been some studies on the effects of lean amine parameters on the performance of the sweetening processes [20], I couldnʼt find a comprehensive research, studying the suggested target parameters for the selected processes.
Feed gas specifications
All the three processes are designed for sweetening the natural gas produced in a gas field having high CO2 /H2S ratio. The sour gas produced in this field has the specifications. It can be seen from the data presented that the natural gas produced in this gas field has high CO2 /H2S ratio, high CO2 content, low H2S content and low pressure. Thus, it is expected that the results of this study would be applicable for sweetening of the natural gas produced in similar gas fields.
In this study, the desirable sweet gas specifications are supposed to be concentrations lower than 1mol% CO2 and lower than 4ppm H2S.
An overview of the three processes
Alkalonomines are widely used for acid gas removal from natural gas [1, 15, 21-26], they are classified to primary amines, secondary amines and tertiary amines based on the number of alkyl groups having bonds with the N atom in the structure of amino group. The most common alkanolamines used are Monoethanolamine (primary), Diethanolamine (secondaray) and methyldiethanolamine (tertiary) [15, 25-29]. Selection of an alkanolamine process for sweetening of natural gas affects the capital and operating costs, energy requirements, sizing of the equipment and in some cases the type of equipment needed for sweetening [25, 27]. The alkanolamines absorb the acid gases from natural gas via reactions (1-2) [17, 30].
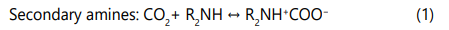
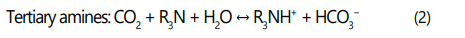
DEA
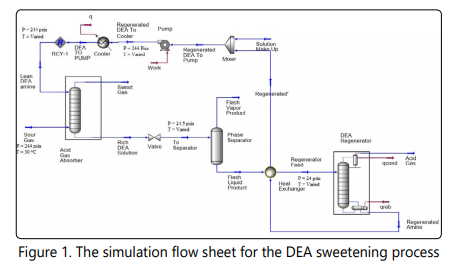
Diethanolamine, abbreviated as DEA is a secondary amine, aqueous solutions of which are used to absorb hydrogen sulfide and carbon dioxide from natural gas [25, 26, 31]. Many products such as COS, CS2 , SO3 and SO2 can catalyze degradation or deactivation of alkanolamine solutions [2, 32]. Due to low reaction rate with CS2 and COS, when considerable amounts of CS2 and COS are present in the sour gas, DEA and other secondary amines are the better choice for natural gas sweetening [25, 26]. DEA solutions are rather unselective and could be used for absorption of either H2S or CO2 from the natural gas [26]. DEA solutions are industrially used with concentrations between 25-40wt% [27, 33]. The DEA sweetening process is simulated using Aspen HYSYS simulator and the different cases of simulation are economically evaluated using aspen economic evaluation (Icarous), the results are compared to that of DGA and MDEA+DEA processes. For simulation of this process, the DBR-Amine property package has been used. The simulation flow sheet is shown in Figure 1. A tray absorber with 20 theoretical stages was used. Also, a tray column with 18 theoretical stages was used for modeling the regenerator column. The pressure of the regenerator varies between 27.5 psia (condenser) to 31.5 psia (reboiler). The rich DEA pressure is reduced to 90 psia in the valve and no pressure drop was assumed in the two phase separator.
DGA
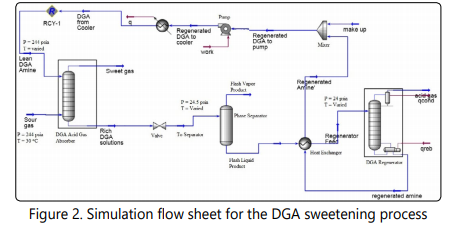
Diglycolamine is a primary amine used for natural gas sweetening. The low vapor pressure of DGA allows using aqueous solutions of this amine in rather high concentrations (40-70 wt%) for natural gas sweetening which results in low amine circulation rates needed for the natural gas sweetening [25, 33]. DGA solutions are particularly effective for treatment of low pressure natural gas. DGA has a tendency to selectively absorb CO2 in presence of H2S [33], however DGA absorbs aromatic compounds which causes the sulfur recovery unit to be more complicated [34], thus, DGA is a good choice for sweetening of natural gas with relatively high CO2 concentration. Based on these statements, DGA is selected as one of the alternatives for sweetening of natural gas with the specifications. In this study a 65wt% aqueous solution of DGA is used for sweetening of the natural gas. Aspen Hysys and Aspen economic evaluation have been used for simulation and economical evaluation of this process. The DBR-Amine property package was used for simulation of this process. The simulation flow sheet for this process is shown in Figure 2. A tray absorber with 20 theoretical stages was used. Also, a tray column with 20 theoretical stages was used for modeling the regenerator column. The pressure of the regenerator is set to 24 psia. The rich DEA pressure is reduced to 25 psia in the valve and no pressure drop was assumed in the two phase separator.
MDEA+DEA
Methyldiethanolamine (MDEA) is a tertiary amine known to have higher selectivity in absorbing H2S in presence of CO2 [27]. The reaction of MDEA with H2S is almost instantaneous while its reaction with CO2 is occurs at lower rates. However, numerous studies show that addition of small amounts of primary or secondary amines to a tertiary amine causes the overall CO2 absorption rate of the process to increase [6, 25, 27, 33, 35-37]. For sweetening of the natural gas, because of relatively high CO2 content in the sour gas, I decided to add 10wt% percent of a secondary amine (DEA) to the solution to increase the CO2 absorption rate of the MDEA process which can make this process a promising process for sweetening of the natural gas described in section 2. The other reason for mixing the suggested amine solutions is to combine the reactivity of the secondary amine and relatively low regeneration energy requirements of tertiary the amine. MDEAʼs typical concentration in aqueous solutions is 30- 50wt% in industrial applications. In this study an aqueous solution of 40wt% MDEA and 10wt%DEA is selected for sweetening the natural gas introduced in section 2 which is one the cases with the best performance regarding absorption of CO2 [6]. Aspen HYSYS is used for simulation of this process and Aspen economic evaluation is used for economically evaluating this process. The DBR-Amine property package is used for simulation of this process. The simulation flow sheet is shown in Figure 3. A tray absorber with 20 theoretical stages was used. Also, a tray column with 20 theoretical stages was used for modeling the regenerator column. The pressure of the regenerator is set to 24 psia. The rich DEA pressure is reduced to 25 psia in the valve and no pressure drop was assumed in the two phase separator.
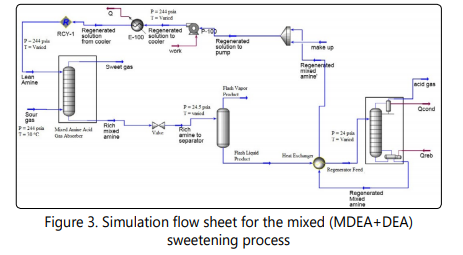
Results and discussion
Simulation results and operating conditions
For each of the three processes, seven different scenarios have been designed for studying the effects of coolerʼs operating parameters on the performance of the three sweetening processes. Each of these scenarios, shows the characteristics of the system at a certain operating condition of the cooler. The coolerʼs duty in each scenario is varied until the lean solution temperature reached the designed value. In each scenario, the processʼs parameters are changed in such a way to reach concentrations lower than 1mol% CO2 and lower than 4ppm H2S for the sweet natural gas.
In simulation of these processes, the minimum temperature approach for all of the heat exchangers has been assumed to be 10°C and the pumpʼs adiabatic efficiency was set at 75%.
After completing the simulation of three processes, for each process these seven scenarios are applied and the process is economically evaluated using aspen economic evaluation v7.3.
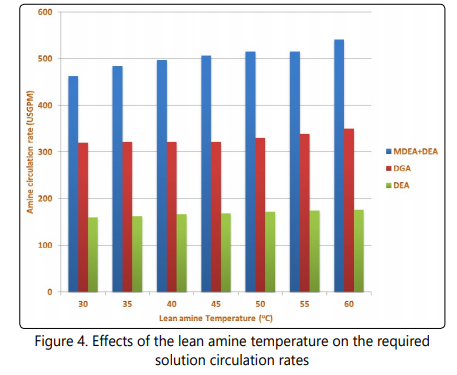
One of the most important characteristics of a sweetening process is the circulation rate (gpm) of the solution [15, 38]. Increasing the solution flow rate causes the capital and operating costs, sizing of equipment and energy requirements of the process to increase [15, 25, 39]. The results of solution flow rate of the processes in different scenarios are shown in Figure 4. It is clear from the data presented in Figure 4 that the amine circulation rate for the mixed amine process is higher than that of DGA and DEA in seven scenarios. It is also shown in Figure 4 that when the lean amine temperature increases, the solution flow rate needed for each process slightly increases and the minimum required solution circulation rate is observed at the lowest lean amine temperature. As mentioned earlier, increasing the solution flow rate in a sweetening plant causes the plantʼs capital and operating costs along with the energy requirements and sizing of the equipment to increase. On the other hand, reducing the temperature of the lean solution requires larger duty of the cooler. This larger duty could be obtained by increasing the contact area of heat exchanger or changing the cooling material, in either way, this change will cause the plantʼs operation to be more expensive. Based on these statements, it seems that there should be an optimum point of operation for the cooler of a sweetening plant. In this study I tried to find this point for three different sweetening processes. As shown in Figure 4, the solution circulation rate for the mixed process is significantly higher than solution circulation rate of the DEA process. This observation is attributed to be due to the fact that methyldiethanolamine selectively absorbs H2S and has lower capacities for absorption of CO2 [25, 26, 40].
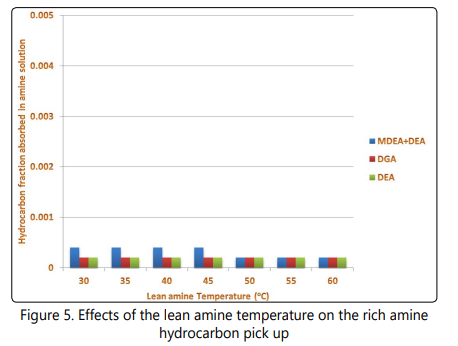
Another important aspect of operation of sweetening processes is the fraction of hydrocarbons absorbed into the solution in the contactor. Based on previous studies, the hydrocarbon co-absorption is mainly a disadvantage of physical and physical-chemical solutions [9, 25, 27, 41, 42], however, I examined this parameter on the three chemical absorption systems to verify the simulation results. As shown in Figure 5, although for the mixed amine process at lower temperatures hydrocarbon pick up is enhanced, the hydrocarbon pick up by the solution remains at a very low rate for different coolerʼs operating conditions in the three processes. The maximum hydrocarbon pick up by the solution in the 21 simulation scenarios was 0.0004 for the mixed amine process. It is also observed in Figure 5 that at temperatures higher than 45o C, the hydrocarbon pick up by the MDEA+DEA process decreases. However the hydrocarbon pick up by the DEA and DGA processes is not affected by lean amine temperature.
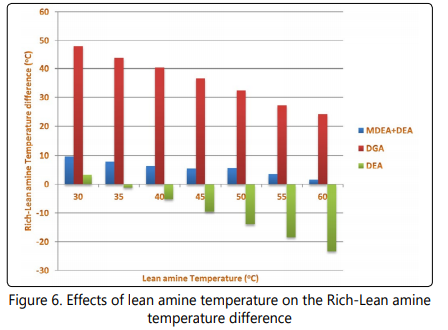
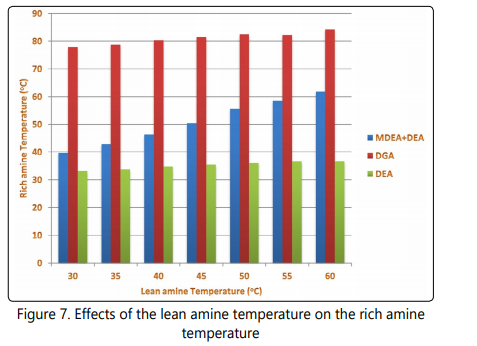
Since the chemical reactions leading to absorption of acid gases into the alkanolamine solutions are exothermic [25, 43- 45], it is expected that the temperature of rich amine be higher than that of the lean amine entering the contactor and the temperature difference between these streams can be a parameter showing the intensity of absorption process in the contactor. In Figure 6 and Figure 7 the temperature difference between rich and lean amine streams, and the rich amine temperatures are shown. Based on the data shown in Figure 7, the temperature of reach amine increases when the lean amine temperature entering the contactor is increased. However, for the three processes the temperature difference between the two streams decreases with increasing the lean amine temperature. For the DEA process, the rich amine temperature is even lower than the temperature of leanamine at lean amine temperatures higher than 35°C. This observation is attributed to be due to higher heat transfer between the cold feed gas (at 21°C) and the lean amine due to increase in temperature difference between feed gas and the lean amine streams.
Another important issue that must be addressed here, is that the rich amine temperature directly affects the energy requirements of the system because the rich amine at the bottom of contactor needs to be regenerated at high temperatures. Thus, when the rich amine temperature is increased, the systemʼs energy requirements (or heat exchangerʼs contact area) will decrease.
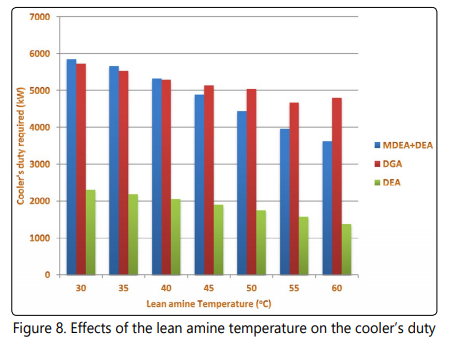
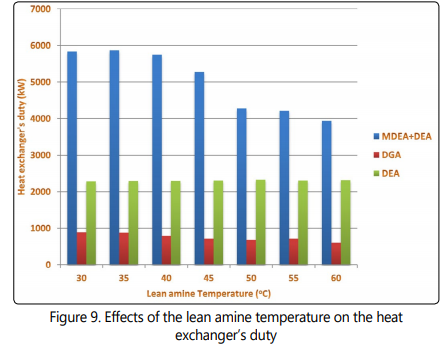
Another important characteristic of the sweetening processes is the energy requirements. The lean amine temperature directly affects the duty that needs to be applied in the cooler. Lean amine temperature also affects the stripperʼs energy requirements and the heat exchanger duty. Figure 8 shows that when the lean solution temperature decreases, the coolerʼs duty increases for the three processes which is an expected observation because the temperature difference around the cooler increases by decreasing the outlet temperature. The minimum cooler duty is observed at the highest lean amine temperature which is in accordance to the expected trend. It is also shown in Figure 9 that the heat exchanger duty follows a reducing trend by increasing the lean amine temperature. Another important observation in Figure 9 is considerably lower heat exchanger duty for the DGA process compared to DEA and MDEA+DEA processes. This observation is because of the fact that the temperature of the rich amine in the DGA process is considerably higher than that of the other two processes. Low cooler and heat exchanger duty of the DEA process are also attributed to be due to lower solution circulation rate of this process compared to the DGA and MDEA+DEA processes.
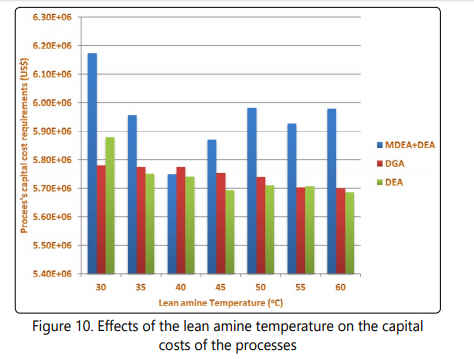
After completing simulation of seven scenarios for each of the processes, each scenario is economically evaluated using aspen economic evaluation. It has been assumed that the projects are about be constructed in 2014. The results are obtained in US$ or US$/year for different scenarios. Parameters such as complexity of the processes, start date and level of instrumentation are taken into account for estimation capital and operating costs of the processes. As shown in Figure 10, based on the results of economic evaluation, the capital costs of the MDEA+DEA process passes through a minimum when the lean amine temperature reaches 40 °C. Also it is clear that with increasing the lean amine temperature from 30 °C to 60°C, the capital costs of the DEA and DGA processes follow a decreasing trend. The lowest process capital cost is obtained when the DEA process is used and the lean amine temperature of this process is the maximum examined temperature and the capital costs of the DGA process are slightly higher than capital costs of the DEA process.
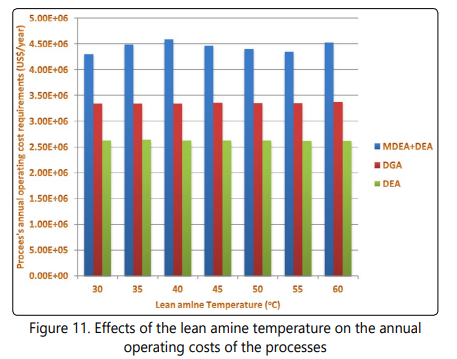
The annual operating cost results of the seven scenarios simulated for each of the processes are shown in Figure 11. According to the data shown in Figure 11, the annual operating costs of the three processes are not strong functions of the lean amine temperature. These observations can be justified by undermining the data shown in Figure 8 and Figure 9. It was mentioned earlier that the stripperʼs reboiler duty doesnʼt vary with changing the lean amine temperature, also, it was mentioned that decreasing the lean amine temperature has a positive effect on the heat exchangerʼs duty and a negative effect on the coolerʼs duty. Based on these information it is concluded that the negative and positive effects of this change are not very steep or that these effects neutralize each other and this the reason that no discernable change in utility costs and subsequently annual operating costs of the system is reported. It is also clear from Figure 11that the annual operating costs and utility costs of the DEA process are lower than that of the DGA and the MDEA+DEA processes.
Considering a life cycle of 25 years for operating the three processes, the dominant costs associated with the processes are the annual operating costs and utility costs. From the data shown in Figure 11 it is observed that the annual operating costs and utility costs of the DGA and DEA processes are not affected by the choice of lean amine temperature, so for these two processes, the lean amine temperature doesnʼt play a crucial part in the costs of the processes. However, for the MDEA+DEA process, the results are more complicated and the annual operating costs of the process donʼt follow a simple trend and the minimum annual operating costs are observed at lean amine temperature of 30°C. Considering a life cycle of 25 years, this temperature shows the best economic performance for this process.
Based on the fore mentioned discussions, there are several advantages in operating the DGA, DEA and MDEA+DEA sweetening processes with higher lean amine temperatures. Lower processʼs capital costs, lower rich amine hydrocarbon pick up in case of MDEA+DEA process and lower coolerʼs duty are obtained by operating the process at higher lean amine temperatures.
An improvement to the results of this research can be investigation of cost and energy requirements of other suitable sweetening processes. Investigating costs and energy requirements of other suitable processes for the sweetening of natural gas with specifications close to the natural gas that i have considered, can be the topic of future studies.
Conclusion
Effects of coolerʼs operating parameters on the performance of three sweetening processes designed for sweetening the natural gas produced in a gas field having high CO2 /H2S ratio (with the specifications described in section 2) have been investigated. DEA, DGA and MDEA+DEA processes have been selected for sweetening the natural gas produced in this gas field. Each of these processes was designed in such a way to reach concentrations lower than 1mol% CO2 and lower than 4ppm H2S for the sweet gas.
Based on the results of this study, for DEA and DGA processes, in the range of lean amine temperature between 30 °C – 60 °C, operating the processes with higher lean amine temperature exhibit several advantages. Lower processʼs capital costs, lower rich amine hydrocarbon pick up in case of MDEA+DEA process and lower coolerʼs duty were obtained by operating the processes at higher values of lean amine temperature. Although the circulation rate of the solution needed to reach concentrations lower than 1mol% CO2 and lower than 4ppm H2S for the sweet gas slightly increased when the lean amine temperature increased, it is recommended to operate the DGA and DEA sweetening processes at higher lean amine temperatures.
An improvement to the results of this research can be investigation of cost and energy requirements of other suitable sweetening processes. Investigating costs and energy requirements of other suitable processes for the sweetening of natural gas with specifications close to the natural gas that I have considered, can be the topic of future studies.
References
- Mokhatab S, Poe WA. Handbook of natural gas transmission and processing: Gulf Professional Publishing; 2012.
- Sanggie FW. Process modeling and comparison study of acid gas removal unit by using different aqueous amines: Universiti Malaysia Pahang; 2011.
- Versteeg GF, Van Swaalj W. Solubility and diffusivity of acid gases (carbon dioxide, nitrous oxide) in aqueous alkanolamine solutions. J. Chem. Eng. Data. 1988; 33(1): 29-l34. doi: 10.1021/je00051a011
- Park SH, Lee KB, Hyun JC, Kim SH. Correlation and prediction of the solubility of carbon dioxide in aqueous alkanolamine and mixed alkanolamine solutions. Ind. Eng. Chem. Res. 2002; 41(6): 1658-65. doi: 10.1021/ie010252o
- Sidi-Boumedine R, Horstmann S, Fischer K, Provost E, Fürst W, et al. Experimental determination of carbon dioxide solubility data in aqueous alkanolamine solutions. Fluid phase equilibria. 2004; 218(1): 85-94. doi: 10.1016/j.fluid.2003.11.014
- Sohbi B, Meakaff M, Emtir M, Elgarni M. The using of mixing amines in an industrial gas sweetening plant. Proceedings of World Academy of Science Engineering & Technology. 2007; 25: 1307-6884.
- Fouad WA, Berrouk AS. Using mixed tertiary amines for gas sweetening energy requirement reduction. Journal of Natural Gas Science and Engineering. 2013; 11: 12-7.
- Freeman SA, Dugas R, Van Wagener DH, Nguyen T, Rochelle GT. Carbon dioxide capture with concentrated, aqueous piperazine. International Journal of Greenhouse Gas Control. 2010; 4(2): 119-24. doi: 10.1016/j.ijggc.2009.10.008
- Rivas O, Prausnitz J. Sweetening of sour natural gases by mixed-solvent absorption: Solubilities of ethane, carbon dioxide, and hydrogen sulfide in mixtures of physical and chemical solvents. AIChE Journal. 1979; 25(6): 975-84. doi: 10.1002/aic.690250608
- Warudkar SS, Cox KR, Wong MS, Hirasaki GJ. Influence of stripper operating parameters on the performance of amine absorption systems for post-combustion carbon capture: Part I. High pressure strippers. International Journal of Greenhouse Gas Control. 2013; 16: 342-50. doi: 10.1016/j.ijggc.2013.01.050
- Polasek JC, Bullin JA, Donnelly ST. Alternative flow schemes to reduce capital and operating costs of amine sweetening units. Energy Processing/ Canada. 1982; 74: 45-50.
- Mores P, Rodríguez N, Scenna N, Mussati S. CO2 capture in power plants: Minimization of the investment and operating cost of the postcombustion process using MEA aqueous solution. International Journal of Greenhouse Gas Control. 2012; 10: 148-63. doi: 10.1016/j.ijggc.2012.06.002
- Bae HK, Kim SY, Lee B. Simulation of CO2 removal in a split-flow gas sweetening process. Korean J. Chem. Eng. 2011; 28: 643-8. doi: 10.1007/ s11814-010-0446-6
- Cousins A, Wardhaugh L, Feron P. A survey of process flow sheet modifications for energy efficient CO2 capture from flue gases using chemical absorption. International Journal of Greenhouse Gas Control. 2011; 5(4): 605-19. doi: 10.1016/j.ijggc.2011.01.002
- Kazemi A, Malayeri M, Shariati A. Feasibility study, simulation and economical evaluation of natural gas sweetening processes–Part 1: A case study on a low capacity plant in iran. Journal of Natural Gas Science and Engineering. 2014; 20: 16-22. doi: 10.1016/j.jngse.2014.06.001
- Ghanbarabadi H, Khoshandam B. Simulation and comparison of Sulfinol solvent performance with Amine solvents in removing sulfur compounds and acid gases from natural sour gas. Journal of Natural Gas Science and Engineering. 2015; 22: 415-20. doi: 10.1016/j.jngse.2014.12.024
- Nuchitprasittichai A, Cremaschi S. Optimization of CO2 capture process with aqueous amines using response surface methodology. Computers & Chemical Engineering. 2011; 35(8): 1521-31. doi: 10.1016/j.compchemeng.2011.03.016
- Øi LE, Bråthen T, Berg C, Brekne SK, Flatin M, et al. Optimization of configurations for amine based CO2 absorption using Aspen HYSYS. Energy Procedia. 2014; 51: 224-33. doi: 10.1016/j.egypro.2014.07.026
- Banat F, Younas O, Didarul I. Energy and exergical dissection of a natural gas sweetening plant using methyldiethanol amine (MDEA) solution. Journal of Natural Gas Science and Engineering. 2014; 16: 1-7. doi: 10.1016/j.jngse.2013.10.005
- Abdulrahman R, Sebastine I. Effect of lean amine temperature on amine gas sweetening: case study and simulation. Chemistry and Technology of Fuels and Oils. 2013; 49: 293-7.
- Blanchon le Bouhelec E, Mougin P, Barreau A, Solimando R. Rigorous modeling of the acid gas heat of absorption in alkanolamine solutions. energy & fuels. 2007; 21(4): 2044-55. doi: 10.1021/ef0605706
- Hoff KA, Juliussen O, Falk-Pedersen O, Svendsen HF. Modeling and experimental study of carbon dioxide absorption in aqueous alkanolamine solutions using a membrane contactor. Industrial & engineering chemistry research. 2004; 43(16): 4908-21. doi: 10.1021/ie034325a
- Homberg OA, Sheldrake CW, Singleton AH. Regeneration of alkanolamine absorbing solution in gas sweetening processes. Google Patents; 1977.
- Kim I, Hoff KA, Hessen ET, Haug-Warberg T, Svendsen HF. Enthalpy of absorption of CO2 with alkanolamine solutions predicted from reaction equilibrium constants. Chemical Engineering Science. 2009; 64(9): 2027-doi: 10.1016/j.ces.2008.12.037
- Kohl AL, Nielsen R. Gas purification: Gulf Professional Publishing; 1997.
- Maddox RN. Gas and liquid sweetening: JM Campbell for Campbell Petroleum series; 1974.
- Association NGPS, Association GPS, Association GP. Engineering data book: Suppliers Association; 1957.
- Littel R, Versteeg G, Van Swaaij WP. Kinetics of CO2 with primary and secondary amines in aqueous solutions —I. Zwitterion deprotonation kinetics for DEA and DIPA in aqueous blends of alkanolamines. Chemical engineering science. 1992; 47(8): 2027-35. doi: 10.1016/0009-2509(92)80319-8
- Camacho F, Sánchez S, Pacheco R, Rubia L, Dolores M, et al. Kinetics of the reaction of pure CO2 with N-methyldiethanolamine in aqueous solutions. International Journal of Chemical Kinetics. 2009; 41(3): 204-14. doi: 10.1002/ kin.20375
- Cummings AL, Smith GD, Nelsen DK. Advances in amine reclaiming–why thereʼs no excuse to operate a dirty amine system. Laurance Reid Gas Conditioning Conference, 2007.
- Erik J. Reactions between Carbon Dioxide and Amino Alcohols. III. Diethanolamine. Acta Chemica Scandinavica. 1956; 10: 747-55.
- Islam M, Yusoff R, Ali B. Degradation studies of amines and alkanolamines during CO2 absorption and stripping system. Engineering e-Transaction. (ISSN 1823-6379) 2010; 5: 97-109.
- Polasek J, Bullin J. Selecting amines for sweetening units. ENERGY PROGRESS. 1984; 4: 146-9.
- Paul DR, Lloyd DR, Eldridge RB. Carbon dioxide removal from natural gas by membranes in the presence of heavy hydrocarbons and by Aqueous Diglycolamine®/Morpholine, 2004.
- Polasek JC, Inglesias-Silva G, Bullin JA. Using mixed amine solutions for gas sweetening. Proceedings of the Annual Convention-Gas Processors Association: Gas Processors Association; 1992. 58.
- Spears ML, Hagan KM, Bullin JA, Michalik CJ. Converting to DEA/MDEA mix ups sweetening capacity. Oil and Gas Journal. 1996; 94: 63-8.
- Mohamadirad R, Hamlehdar O, Boor H, Fattahi-Monavar A, Rostami S. Mixed amines application in gas sweetening plants. Chemical Engineering Transactions. 2011; 24: 265-70. doi: 10.3303/CET1124045
- Chan CW, Tontiwachwuthikul P. A decision support system for solvent selection of CO2 separation processes. Energy conversion and management. 1996; 37: 941-6.
- Astaria G, Savage DW, Bisio A. Gas treating with chemical solvents, 1983.
- Mandal BP, Biswas A, Bandyopadhyay S. Selective absorption of H2S from gas streams containing H2S and CO2 into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol. Separation and purification technology. 2004; 35(3): 191-202. doi: 10.1016/S1383- 5866(03)00139-4
- Henni A, Tontiwachwuthikul P, Chakma A. Solubility study of methane and ethane in promising physical solvents for natural gas sweetening operations. J. Chem. Eng. Data. 2006; 51(1): 64-7. doi: 10.1021/je050172h
- Nassar VL, Bullin JA, Lyddon LG. Solubility of Hydrocarbons in Physical Solvents.
- Zare Aliabad H, Mirzaei S. Removal of CO2 and H2S using Aqueous Alkanolamine Solusions. International Journal of Chemical and Molecular Engineering. 2009; 3.
- Kim YE, Lim JA, Jeong SK, Yoon YI, Bae ST, et al. Comparison of carbon dioxide absorption in aqueous MEA, DEA, TEA, and AMP solutions. Bull Korean Chem Soc. 2013; 34(3): 783-7. doi: 10.5012/bkcs.2013.34.3.783
- Jonassen Ø, Kim I, Svendsen HF. Heat of Absorption of Carbon Dioxide (CO2 ) into Aqueous N-Methyldiethanolamine (MDEA) and N, N-Dimethylmonoethanolamine (DMMEA). Energy Procedia. 2014; 63: 1890-902. doi: 10.1016/j.egypro.2014.11.198



