Research Article
Thermodynamic properties Methyl- and Ethyladamantanes
Atyrau Oil and Gas University, Atyrau, Kazakhstan
*Corresponding author: Amanzhan Saginayev, Professor, Atyrau Oil and Gas University, Atyrau, Kazakhstan, E-mail: asaginaev@mail.ru
Received: November 17, 2017 Accepted: December 7, 2017 Published: December 13, 2017
Citation: Saginayev AT, Kursina MM, Gilazhov EG. Thermodynamic properties Methyl- and Ethyladamantanes. Int J Petrochem Res. 2017; 1(2) 101-104. doi: 10.18689/ijpr-1000118
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The thermodynamic characteristics of the compounds and their total energy, transformation energies, entropies of transformations, and normal vibration frequencies have been calculated. It is shown that the calculated free Gibbs energies for the formation of isomerization products of per hydroaromatic hydrocarbons are in qualitative agreement with the experimental data of isomerate products.
Key words: adamantane, methyladamantanes, ethyladamantanes, DFT calculation.
Introduction
In the Gulf of Mexico found tiny black diamonds- according to scientists, they were formed from crude oil. These diamonds consist of a force of several dozen carbon atoms, which is less than one billionth of a carat. However, similar hydrocarbons of a diamondlike structure can also find practical application. Their artificial analogues are already used in medicines intended for the treatment of Parkinsonʼs disease and viral infections [1, 2]. In addition, they can find application in the field of nanotechnology [3]. Diamond materials are derivatives of the saturated hydrocarbon adamantane, which was found in oil as far back as 1933. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the crystal lattice of diamond. Numerous adamantane molecules can join together, forming larger diamondoids.
When a large number of such molecules are combined, a diamond is formed-a characteristic regular lattice consisting of carbon atoms.
A group of scientists from ChevronTexacoʼs research division, led by Jeremy Dhala, discovered diamondoids consisting of several (up to 11) adamantane molecules in the oil deposits raised from the bottom of the Gulf of Mexico.
Before, under laboratory conditions, it was not possible to combine together more than four such molecules.
It is completely incomprehensible how diamondoids can be formed from hydrocarbon chains, of which oil consists. Perhaps, they are formed during reactions with methane, the catalyst in which the minerals that make up the clay are. If this is so, nothing prevents them from continuing their growth further, reaching quite sizeable dimensions. It was also found out that diamondoids can form black agglomerations with tiny crystals of diamonds called black technical diamonds. The latter, apparently, were formed not in the conditions of high temperatures and pressure, like ordinary diamonds. A number of scientists believe that they could form in space and get to Earth together with a meteorite substance.
The adamantane molecule has a high degree of symmetry. Some elements of symmetry of adamantane are preserved even when one or more substituents are introduced in the position of the nucleus.
Adamantane and its derivatives have been the object of many studies, both experimental and theoretical.
The molecular structure of adamantane was studied by gas-phase electron diffraction [4], ionization electron spectroscopy [5], photoelectronic spectroscopy [6], electron spin resonance [7], quantum calculations of ionization potentials (PI) and electron affinity (SE) [8].
The aim of this work is to conduct experimental studies and quantum chemical calculations by the method of functional of the energy from the electron density the DFT B3LYP/6-31G*, to study the structure and thermodynamic properties of alkyladamantanes composition C11-C13.
Experimental
Methyladamantanes are of great interest both for use as an artificial calculation field, and for determining the strain energy within this system. Schleiere and his colleagues calculated the strain energies for adamantane and 1,3,5,7-tetramethyladamantane (-6.9 kcal / mol and 5.0 kcal / mol, respectively) [9].
The data of the exact standard enthalpy of formation of these compounds plays a decisive role in the estimation of calculation methods.
The thermodynamic stability of some alkyladamantanes was determined by calculation and experimental methods, and then compared with each other [10].
Alkyladamantanes of the composition C12H20 are obtained from perhydroacenaphthene upon passage of the latter over the alumina catalyst in a flow type plant with a metal reactor [11-14]. The products of this reaction include 1,3-dimethyladamantane, trans-1,4-dimethyladamantane, cis-1,4-dimethyladamantane, 1,2-dimethyladamantane, 1-ethyladamantane and 2-ethyladamantane. As is known, alkyladamantanes C13H22 are usually obtained by isomerization of perhydrofluorene [11,12, 15]. From isomerizate are allocated 1,3,5-trymethyladamantane, cis-1,3,6-trymethyladamantane, trans-1,3,6-trymethyladamantane, cis-1,3,4-trymethyladamantanes, trans-1,3,4-trymethyladamantane, 1,2,6-trymethyladamantane, 1,2,8-trymethyladamantan, 1-methyl3-ethyladamantane, cis-1-methyl-4-ethyladamantanes, trans1-methyl-4-ethyladamantanes.
Calculation results and Discussion
To minimize the errors in the calculations, 1- and
2-methyladamantanes, 2,2-dimethyladamantane, 1,3,5-
trimethyladamantane were purified by standard methods (recrystallization, vacuum sublimation) before burning in the
calorimeter, and 1,3-dimethyladamantane was purified by
repeated fractional distillation under low pressure. Was used
1 cm3
of water was placed into the calorimetric bomb of
internal volume 0.1 dm3
with calibration and additional
equipment and then oxygen pressure up to 30 atmospheres
at 298.15 К was established. The vapor pressures of all the solid compounds were
measured in a glass Bourdon gauge that was nulled against a
mercury manometer. The vapor pressures of these compounds
were fitted by least squares to the equation
log10 (p/Torr) = A/T + B (1)
and the enthalpy of sublimation was calculated assuming
no vapour imperfection and negligible solid volume as [16]
∆Hsub = - RAln10 (2)
The vapor pressure of 1,3-dimethyladamantane is determined by semi microebuliometric method [17].
The results of the calculations are presented in Table 1.
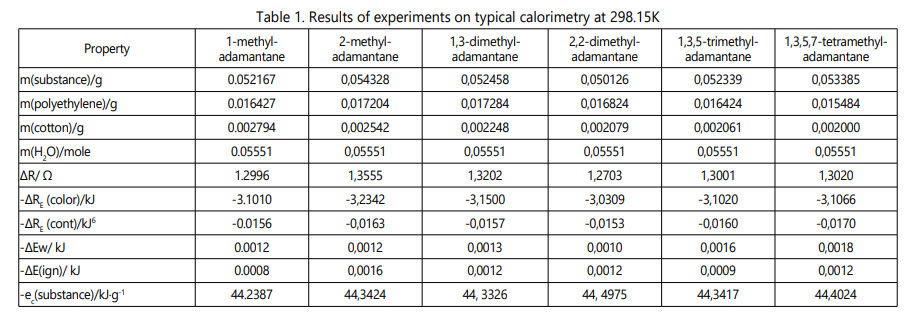
The combustion reaction is described by the equation:
CaHb + (a+b/4) O2
= aCO2 + 1/2bH2O (3)
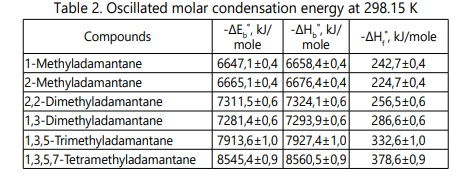
Derivatives from the standard molar combustion energy ∆Eb˚, the standard molar enthalpy of combustion ∆Hb˚ and the molar standard enthalpy of formation of ∆Hf˚ compounds are presented in Table 2.
Derivatives from the sublimation enthalpies of
methyladamantanes are presented in Table 3. The values of
Tm are taken from the previously studied mean temperatures. The standard enthalpy of sublimation ∆Hs˚ (298.15 K) is
determined by the equation:
∆Hs˚ (298.15К) = ∆Hs
˚(Тm) +(298.15 К – Тm) (Сp˚(gas)-
Сp˚(solid)) (4)
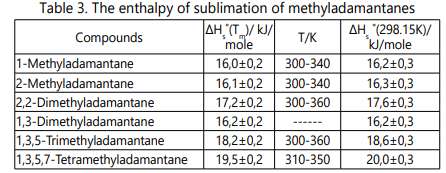
Substitution of methyl groups on tertiary carbon atoms of adamantane nuclei increases thermochemical stability.
We performed quantum-chemical calculations using the energy-functional method from the electron density of DFT B3LYP/6-31G* perhydroacenaphthene, perhydrofluorene and the products of their transformations [18, 19]. Optimization of the geometric structure of molecules and calculation of the frequencies of normal vibrations were carried out using atomic bases 6-31G*. Calculations were performed using the GAUSSIAN-98 program [20]. DFT B3LYP is a combination of the Charter-Fock method and the density functional theory using the gradient-corrected Beck function with three parameters (B3) [21] and the Li-Yang correlation functional series (LYP) [22]. For each molecule, the geometric arrangement of atoms was optimized using analytical calculation methods. By calculating the frequencies of normal vibrations using the second derivatives, it was confirmed that the stationarity points determined in the optimization of geometry are energy minima.
Table 4 shows the calculated electronic characteristics of the calculated molecules: the energy of the boundary orbitals (Ehomo, Elumo), dipole moments (µ), zero-point energy (ZPC) and entropy (S).
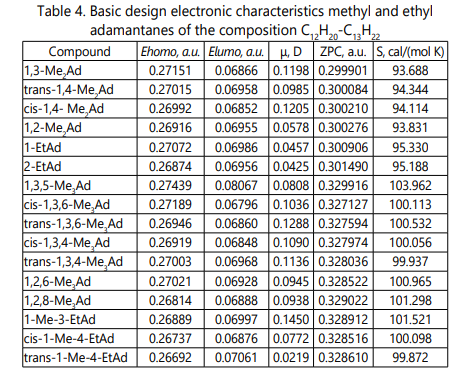
Table 5 shows the calculated basic energy characteristics
of compounds: the values for the total energy Et, the total
energy with allowance for zero point energy Ezpc, the total
energy with correction for enthalpy EH, and the total energy
with correction for Gibbs free energy EG in atomic units of
energy. Below are the formulas for determining these
thermodynamic quantities [21]:
Ezpc = Et+ ZPC, (5)
EH = Et+ ZPC + Evib + Erot + Etrans, (6)
EG = EH – TS, (7)
where, Evib is the vibrational motion energy, Erot is the energy of the rotational motion, Etrans is the translational motion energy, S is the entropy and T is the Kelvin temperature.
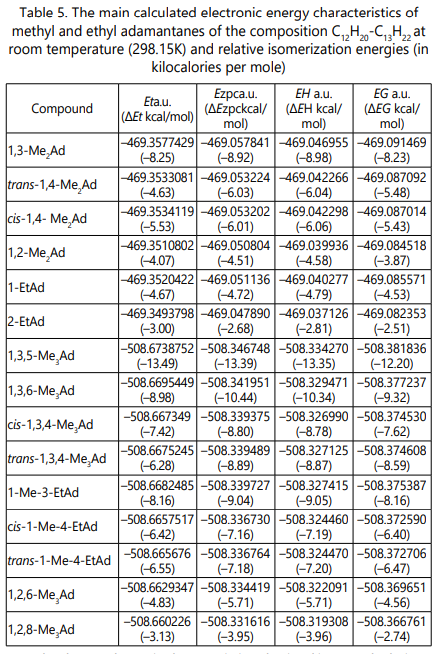
The thermodynamic characteristics obtained in our calculations are in excellent qualitative agreement with experimental data on the isomerization of perhydroacenaphthene [1].
1,3-Dimethyladamantane as the product of isomerization has the greatest of all the other alkyladamantanes of the C12H20 composition with thermodynamic stability; it has the lowest values of Et, Ezpc, EH, EG. The yield of 1,3-dimethyladamantane is up to 80%. The cis and trans isomers of 1,4-dimethyladamantane have practically the same values of all the thermodynamic characteristics that we calculated. For these isomers, the isomerase composition is the same (about 4%).The stability of 1,2-dimethyladamantane is lower than the stability of 1,3-adamantane and 1,4-adamantanes, which also agrees with the experiment. 1-ethyladamantane is more stable from calculations than 2-ethyladamantane. The experimental yields of these products are consistent with our calculations.
The thermodynamic characteristics obtained from our calculations are in excellent qualitative agreement with the experimental data on the isomerization of perhydrofluorene [1]. 1,3,5-Trimethyladamantane as the isomerization product has the greatest of all the other alkyladamantanes of the C13H22 composition with thermodynamic stability; it has the lowest values of Et, Ezpc, EH, EG. The yield of 1,3,5-trimethyladamantane is up to 50%. The cis and trans isomers of 1,3,6-trimethyladamantane and 1,3,4-trimethyladamantane have practically the same values of all the thermodynamic characteristics that we calculated. For these isomers, the isomerizate composition is approximately the same (3 and 4%, respectively). The stability of 1-methyl-4- ethyladamantanes is lower than the stability of 1-methyl-3- ethyladamantane, which also agrees with the experiment. 1,2,6- and 1,2,8-trimethyladamantanes are not stable from the calculations. The experimental yields of these products are consistent with our calculations.
Thus, the results of the calculations presented above agree with the previously published experimental data that the number of different isomers of methyl- and ethyladamantane formed in the isomerization of perhydroaromatic hydrocarbons is due to the difference in their thermodynamic stability. It has been established experimentally that the reaction is an equilibrium process.
References
- Bagrii EI. Adamantanes: Synthesis, Properties, and Application [in Russian]. Nauka, Moscow, 1989.
- Zefirov NS, Moiseev IK. Panorama of Modern Chemistry in Russia: Progress in Adamantane Chemistry.Ed. [in Russian] by Khimiya, Moscow, 2007.
- Bagrii EI, Safir RE. Arinicheva YA. Methods of the functionalization of hydrocarbons with a diamond-like structure. Pet. Chem. 2010; 50(1): 1-16. doi: 10.1134/S0965544110010019
- Hargittai I, Hedberg K. In Molecular Structures and Vibrations. Ed. Elsevier. 1972; 340-57.
- Tian SX, Kishimoto N, Ohno K. Two-Dimensional Penning Ionization Electron Spectroscopy of Adamantanes and Cyclohexanes: Electronic Structure of Adamantane, 1-Chloroadamantane, Cyclohexane, and Chlorocyclohexane and Interaction Potential with He*(23sup S). J. Phys. Chem. A. 2002; 106(28): 6541-6553. doi: 10.1021/jp014302e
- Kruppa GH, Beauchamp JL. Energetics and structure of the 1- and 2-adamantyl radicals and their corresponding carbonium ions by photoelectron spectroscopy. J. Amer. Chem. Soc. 1985; 108(9): 2162-9. doi: 10.1021/ja00269a007
- Kira M, Akiyama M, Ichinose M, Sakurai H. ESR study of 2-substituted 2-adamantyl radicals. Configuration and long-range hyperfine interaction. J. Am. Chem. Soc. 1989; 111: 8256-8262.
- Rienstra-Kiracofe JC, Tschumper GS, Schaefer HF. Atomic and molecular electron affinities: photoelectron experiments and theoretical computations. Chem. Rev. 2002; 102(1): 231-82.
- Engler EM, Andose JD, Schleyer PVR. Critical evaluation of molecular mechanics. J. Am. Chem. Soc. 1993; 115(19): 8005-8025.
- Saginayev AT, Apendina AK. Vestnik of Atyrau Institute of Oil and Gas.10. 125, 2006.
- Bagrii EI, Sanin PI. USSR Inventorʼs Certificate No. 239302. 1971: Byull. Izobret. No. 25, 241: 1971.
- Bagry EI, Sanin PI. US Patent No. 3637876, 1972.
- Bagry EI, Sanin PI, Dolgopolova TN. Neftekhimiya9, 353, 1969.
- Saginayev AT. News of science of Kazakhstan. 2004.
- Schneider A. Reaction of tricyclic perhydroatromatic hydrocarbons. US Patent No. 3128316, 1964
- Steele WV, Watt I. The standard enthalpies of formation of adamantanoid compounds 4. Methyladamantanes. J. Chem. Thermodynamics. 1977; 9: 843-49.
- Boyd RH, Sanwai SN, Shary-Tehrany S, McNally D. The thermochemistry, thermodynamic functions, and molecular structures of some cyclic hydrocarbon. J. Chem. Phys. 1971; 75: 1264.
- Borisov YA, Saginayev AT. Calculation of geometric structure, electronic characteristics, vibration frequencies, and thermodynamic properties of C12H20 alkyladamantanes. Pet. Chem. 2014; 54(4): 270-74. doi: 10.1134/ S0965544114040021
- Borisov YA, Saginayev AT, Bagrii EI. Geometric structure, electronic structure, and some thermodynamic properties of C13H22 trimethyl- and ethylmethyladamantanes. Pet. Chem. 2016; 56(2): 166-70. doi: 10.1134/ S0965544116020043
- Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 98, Revision A.5, Pittsburgh, 1998.
- Becke AD. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993; 98: 1372. doi: 10.1063/1.464304
- Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlationenergy formula into a functional of the electron density. Phys Rev B Condens Matter. 1988; 37(2): 785-89.



