Research Article
Halogenated Wastes Safe Disposal: Polychlorinated biphenyls
1 1 School of Engineering, Australian College of Kuwait, P.O. Box 1411, Safat 13015, Kuwait
2Sustainable and Renewable Energy Engineering Department, University of Sharjah, P.O.Box 27272, Sharjah, United Arab Emirates
*Corresponding author: Mamdouh El Haj Assad, Sustainable and Renewable Energy Engineering Department, University of Sharjah, P.O.Box 27272, Sharjah, United Arab Emirates, E-mail: massad@sharjah.ac.ae
Received: April 4, 2017 Accepted: May 2, 2017 Published: May 10, 2017
Citation: Bani-Hani EH, El Haj Assad M. Halogenated Wastes Safe Disposal: Polychlorinated biphenyls. Int J Petrochem Res. 2017; 1(2): 76-78. doi: 10.18689/ijpr-1000113
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The aim of this work is to have a review of some products where halogens are used
in the manufacturing process along with the safe disposal methods of its wastes. Different halogenated compounds are introduced. The theoretical results are validated
by comparing them with experimental results. Results show that incineration is a safe
disposal method of polychlorinated Biphenyls (PCBs). The incineration of PCBs in rotary
kiln is shown based on theoretical and experimental studies. Where energy and material
balances are applied to rotary kiln incineration to derive the theoretical model. The
results from the theoretical model is validated against experimental results. The disposal
method by incineration for other halogenated compounds is presented. Different
models representing the combustion of other halogenated components are analyzed. It
is shown that incineration is the safe disposal method for halogenated compounds at
temperatures around 1200 K to avoid the formation of toxic gas emissions like furan and
dioxin. The theoretical results are comparable with the experimental data of the
incineration process.
Key words: Polychlorinated biphenyl; halogenated compounds; Incineration
Introduction
The halogens group in the periodic table consisting of five elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). In the modern IUPAC nomenclature, this group is known as group 17. Halogens react with metals to produce a wide range of
salts, including calcium fluoride, sodium chloride, silver bromide and potassium iodide. They have many industrial applications such as in electrical equipment and brominated
flame retardants [1]. Halogenated wastes come from Laboratory wastes containing
various hazardous chemical reagents and reactants. They are often discharged from
experiments, tests, or analyzing processes [2]. Famous halogenated components are
Polychlorinated biphenyls (PCBs) which are a class of organic compounds. PCBs classified
as persistent organic pollutants (POPs) that retard the biodegradation process and
stayed for long time in the environment. PCBs contain 1 to 10 chlorine atoms attached
to biphenyl and a general chemical formula of C12H10-xClx most PCBs were manufactured
as cooling and insulating fluids for industrial transformers and capacitors stabilizing
additives in flexible PVC coatings of electrical wiring and electronic components [3].
Studies show concentrations of polybrominateddiphenylethers (PBDEs) in the North
American environment were increasing over the last twenty five years and are among
the highest concentrations in the world [4] [5] [6] [7] as this product is banned in Europe
and many countries in the world but it is still in use in north America.
For all of the above mentioned reasons, a need for safe
disposal methods is investigated. One of the effective methods
is thermal treatment. Incineration is one of the thermal
treatment methods in waste management of halogenated
wastes and substances, in which the wastes are combusted at
high temperatures. Although, there are many other methods
of remediation technologies [8] Such as chemical treatment, however, there are many risks incorporated with these
methods. The risk of creating extremely toxic dibenzodioxins
and dibenzofuransis high through partial oxidation particularly
due to high thermodynamic stability of PCBs.
other risks are similar to other treatment methods are all
degradation mechanisms are difficult to sustain. Intentional
degradation as a treatment of unwanted PCBs generally
requires high heat or catalysis. Environmental and metabolic
degradation generally proceeds quite slowly relative to many
other compounds.
In this work,the incineration mechanismof PCBs as the
best safe disposal method will be presented as an example of
halogenated compounds. Then the mechanism and model
are validated against an experimental published data of
similar chlorinated compounds.
Chemical equilibrium reaction of PCBs
Theoretical mechanism of the incineration of hazardous substances is difficult to anticipate as they have wide variations in the constituents. PCBs for example include the number of chlorine atoms varies from (1 to 10). A general stoichiometric relationship can be used to describe the structure of the feed stock to the incinerator [9] as the chemical structure of PCBs showed hydrogen and carbon as main component where chlorine atoms are added, and other chemicals formed minor composition in the structure or it can mix with the oils after use such as sulphur keeping in mind that the incineration process occurs not only for the pure oil but for its wastes:
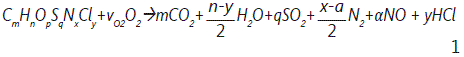
However, the following general expression and chemical reaction for PCBs incineration for this studyis considered as:
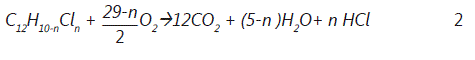
Where in the case of the heterogeneous combustion of carbon mono-oxide (CO) is assumed to be the main oxidation product of carbon. The total combustion to CO2 takes place in the gas phase. Furthermore, waste compounds containing nitrogen are decomposed to give N2 and NO according to the following equation [10]:

Which is used to evaluate α (in equation 1), which is the fraction of kiln temperature and excess oxygen. The resulting equations are solved simultaneously to get the required temperatures, flow rates, energy produced, and gas phase concentrations.
In previous work [3], studied the incineration process of PCBs wastes in a rotary kiln by a dynamic model consisted of a set of nonlinear equations comes from applying material and energy balances under unsteady state conditions. In another study [9] showed the model of PCBs incineration and validation. The dynamic model accounts for the variations in composition of PCBs (number of chlorine atoms) and the process conditions such as excess air, temperature, and pressure of operation. Then the equations are solved using MATLAB. Finally, the results are presented and discussed. Equation 4 which is derived by Bani-Hani et al [3] shows the energy change E with process parameters such as temperature T, mass M, time t, and heat capacities Cv.
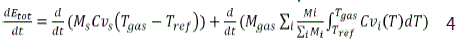
Model Validation
The incineration of PCBs requires high temperature to avoid the production of unwanted toxic byproducts such as furan and dioxin.
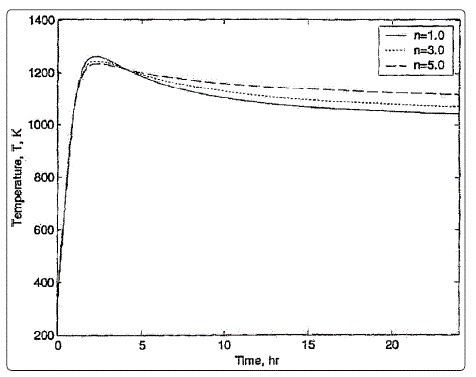
Figure 1: Temperature inside rotary kiln with different number of chlorine atoms and using 10% excess of air where n is the number of chlorine atoms
Previously published data [3] is shown in figure 1. Figure
1 shows an increase in the temperature of incineration with
time till it reaches a steady state value. The temperature is
higher for higher number of chlorine atoms, which is attributed
to the need of more energy to break the bond of chlorine
connected to the structure. It is shown in figure 1that the
temperature is above 1000 K. The obtained results have been
validated against published experimental data and found in
good agreement.
The modeling of PCBs incineration is based mainly on
experimental studies and pilot plants, on the other hand
software or numerical solution needs some assumptions and
approximations. Thus a comparison between theoretical
results and experimental data is necessary. Figure 2 shows a
comparison for some of chlorinated wastes incinerated in
rotary kilns. The results obtained from the theoretical analysis
are comparable to the published experimental data regardingthe temperature of the combustion that reaches around 1200
K. the difference between the two results is attributed to
variations in the component structure.
In their experimental work [11] investigated possible
combustion mechanisms of chlorinated compounds such as
dichloromethane (DCM) and chlorobenzene (MCB).
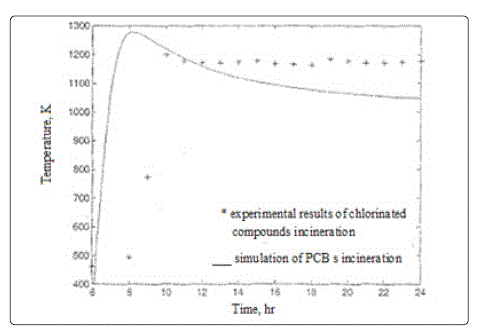
Figure 2: Temperature inside rotary kiln from the current model and compared with experiments
It is found that, to achieve a complete destruction of DCM and MCB, the temperature of combustion should be above 900°C (1173 K) and this is causing the degree of conversion of chlorine compounds very high(92-100%) [12]. Also adjusting the temperature to 300 °C, 600 °C, and 900 °C when investigating the formation of several chlorinated compound classes helps to estimate the effect of temperature on PVC combustion. The literature reviewed showed a comparable results with the model of PCBs combustion temperature which is 1200 K.
Conclusion
Incineration of halogenated wastes is a safe disposal
method. However, the uncontrolled incineration of
polyhalogentaed compounds is a main source of hazardous
gas emissions. Thermal treatment of waste containing flame
retarding products is a source of PBDEs to North American air. Results available in literature showed that the suitable
temperature of incineration is about 1200 K to reduce
emissions of polychlorinated dioxins and furans (PCDDs/Fs).
A temperature of 1200K is recommended to incinerate
some halogenated compounds like PCBs. This temperature
depends on the number of chlorine atoms and the amount of
excess air. To control the air quality additional tools can used
such as gas emissions control systems.
References
- Zhang S, Yub Y. Dechlorination behavior on the recovery of useful resources from WEEE by the steam gasification in the molten carbonates. Procedia Environmental Sciences. 2016; 31: 903-910. doi: 10.1016/j. proenv.2016.02.108
- Wu J, Ta-Chang L, Lin-Chi W, et al. Memory Effects of Polychlorinated Dibenzo-p-dioxin and Furan Emissions in a Laboratory Waste Incinerator. Aerosol and Air Quality Research. 2014; 14(4): 1168-178. doi: 10.4209/ aaqr.2013.05.0179
- Bani-Hani E, Hammad M, Matar A, et al. Numerical analysis of the incineration of polychlorinated biphenyl wastes in rotary kilns. Journal of Environmental Chemical Engineering. 2016; 4(1): 624-632. doi: 10.1016/j. jece.2015.12.006
- Hale R, Alaee M, Manchester-Neesvig J, et al. Polybrominateddiphenyl ether flame retardants in the North American environment. Environment International. 2003; 29(6): 771-779. doi: 10.1016/S0160-4120(03)00113-2
- Schecter A, Johnson-Welch S, Kuang C, et al. Polybrominated Diphenyl Ether (PBDE) levels in livers of U.S. human fetuses and newborns. Journal of Toxicology and Environmental Health. 2007; 70(1): 6. doi: 10.1080/15287390600748369
- Schecter A, Päpke O, HarrisT, et al. Food and Estimated PBDE Dietary Intake by Age and Sex. Environmental Health Perspectives. 2006; 114(10): 1515-1520. doi: 10.1289/ehp.9121
- Schecter A, Pavuk M, Päpke O, et al. Polybrominated diphenyl ethers (PBDEs) in U.S. mothersʼ milk environ. Environmental Health Perspectives. 2003; 111(14): 1723-1729. doi: 10.1289/ehp.6466
- Hu X, Jianxin Z, Qiong D. Environmental life-cycle comparisons of two polychlorinated biphenylremediation technologies: Incineration and base catalyzed decomposition. Journal of Hazardous Materials. 2011; 191(1-3): 258-68. doi: 10.1016/j.jhazmat.2011.04.073
- Bani-Hani E, Hammad M, Matar A, et al. Analysis of Polychlorinated Biphenyl Wastes Incineration in Rotary Kilns Model Development and Validation. Int. J MechSyst Eng. 2015; 1: 103. doi: 10.15344/ijmse/2015/103
- Rovaglio M, Manca D, Biardi G. Dynamic Modeling of Waste Incineration Plants with Rotary Kilns: Comparisons between experimental and simulation data. Chemical engineering science. 1998; 53(15): 2727-742. PII: S0009– 2509(98)00081– 5. doi: 10.1016/S0009-2509(98)00081-5
- Olek M, Baron J, Zukowski. Thermal decomposition of selected chlorinated hydrocarbons during gas combustion in fluidized bed. Chem Cent J. 2013; 7: 2. doi: 10.1186/1752-153X-7-2
- Kim K, Hong K, Ko Y, et al. Emission characteristics of PCDD Fs, PCBs, chlorobenzenes, chlorophenols, and PAHs from polyvinylchloride combustion at various temperatures. J Air Waste Manag Assoc. 2004; 54: 555-562. doi: 10.1080/10473289.2004.10470925



