Review Article
Application of Ionic Liquids in the Upstream oil Industry-A Review
Petroleum Engineering Department, Khalifa University of Science and Technology, Petroleum Institute, P.O. Box 2533, Abu Dhabi, United Arab Emirates
*Corresponding author: Bisweswar Ghosh, Petroleum Engineering Department, Khalifa University of Science and Technology, Petroleum Institute, P.O. Box 2533, Abu Dhabi, United Arab Emirates, E-mail: bghosh@pi.ac.ae
Received: March 1, 2017 Accepted: April 24, 2017 Published: April 29, 2017
Citation: Turosung SN, Ghosh B. Application of Ionic Liquids in the Upstream oil Industry-A Review. Int J Petrochem Res. 2017; 1(1): 50-60. doi: 10.18689/ijpr-1000110
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Ionic liquids are gaining much attention as green chemicals due to their unique properties such as extremely low vapor pressure, high thermal and chemical stability, low toxicity and possibility of tuning their cation and anion moieties to make them task-specific. In the chemical industry, they are well established for innumerable processes and applications and several review articles are available. Contrary to this the applications of ionic liquids in the upstream petroleum industry is relatively new and not enough review articles are available. This article discusses the applications of ionic liquids in areas such as CO2 capture and separation which could be used for enhanced oil recovery and sequestration, heavy crude oil upgradation and viscosity reduction, interfacial tension and surface tension reduction in order to supplement surfactants in chemical enhanced oil recovery and asphaltene dispersion and inhibition during crude oil production and surface transportation. It aims to highlight the wide range of application possibilities of ionic liquids in the upstream sector of the petroleum industry and to encourage further research into some of these areas in order to develop environmentally friendly alternatives to current processes.
Keywords: Upstream; electrochemistry; catalysis; Spectroscopy
Introduction
Ionic liquids (ILs) are compounds of ionic-covalent crystalline structures containing
only ions at room temperature [1]. They are mainly salts but differ from molten salts as
they have a melting point ranging from -100°C to 200°C [2] [3], whereas molten salts
generally have higher melting points. ILs display unique properties originating from a
complex interplay of columbic, hydrogen bonding, and van-der-Waals interactions of
their ions in liquid state [4].
ILs contain a functional group either as an anion or a cation, or both which incorporate
a specific property, either physical or chemical viz. melting point, solubility, hydrophobicity, viscosity, reactivity etc. The desired properties can be achieved through structural
modifications of either the anion, the cationic core, or the substituentʼs on the anion or
cation. If needed there exists the possibility of fine-tuning the ions which provides the means
of diverse applications of ILs [5] [6]. Their physical and thermal properties strongly depend
on the cation and anion species as well as on the alkyl chain length on the cation [7].
In recent years, many room temperature ILs or RTILs (ILs which are liquid at or below
room temperature) are developed and got tremendous attention as potential “green” solvents due to their environment friendly and renewable characteristics, though not all
ionic liquids are environmental friendly [8].
Due to these wide range of advantages, ILs are preferred for applications in organic
synthesis, catalysis, electrochemistry, electro catalysts, chemical separation, solid support, chemical fixation of CO2, nanoparticle formation, and metal extraction, in addition to
their electrochemical stability and high ionic conductivity at room temperature [9].
Ionic liquids can be synthesized in an endless number of
ways such as metathesis of a halide salt with ammonium salt
of the desired anion, combining halide salt with a halide metal
and using nitric acid to neutralize aqueous solution of the
amine. The Figure 1 below shows a typical process for
preparation of ILs.
Figure 1: Typical process for preparing ionic liquids [10].
The various eccentric properties of ILs make them very
attractive for use in different applications. The density of ILs is
generally more than water and increases with increasing
molecular weight of its anion. Furthermore, this property
decreases with increasing alkyl chain length in the cation, thus
density can be modulated as per requirement.
ILs are generally more viscous than other solvents and
show an increase in viscosity as the length of the alkyl chain
increases. In addition, ILs have low melting points due to the
contribution from both cations and anions present. As the size
of anion or cation increases the melting point decreases.
ILs are considered thermally stable as most of them can
sustain up to 500°C temperature for a short period of time. Some others may decompose at this temperature. Also, the
surface tension of ILs is lower than water but generally higher
than other organic solvents. The surface tension of ILs is
affected by the size of their alkyl chain length, as the alkyl
chain length increases the surface tension decreases.
Another very important property of the ILs is their negligible
vapor pressure. This causes them not to evaporate as they are
exposed to higher temperature, particularly in solvent extraction
processes. Additionally, this low vapor pressure is sustained even
at high temperature. Another interesting property of ILs is their
large electrochemical window (5-6 V) in comparison with water (1.23 V) [11]. This is shown in Figure 2 below.
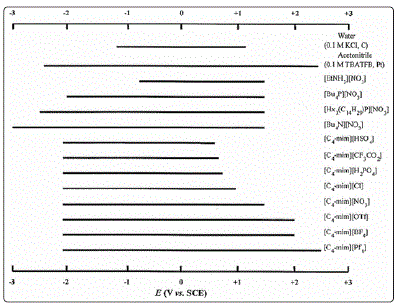
Figure 2: Electrochemical window for ILs [11]
Not enough studies are conducted on the toxicity of ILs, however due to many of their favorable attributes they are considered as green solvents. Table-1 below shows comparison between aqueous amine solvents and ionic liquids andTable-2 compares the solvent properties of ionic liquids with common organic solvents [12] [13] [14].
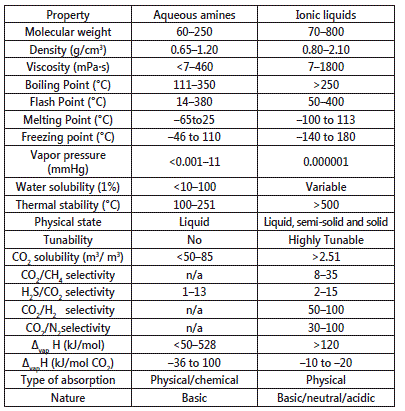
Table 1: Comparison between different properties of ILs and Aqueous amines [12]
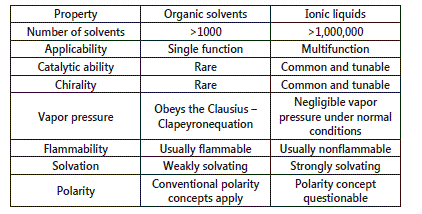
Table 2: Comparison of organic solvents with Ionic liquid [11]
Applications of ILs
With the aforementioned properties, there have been
extensive studies on different applications for ILs. These
applications include:
• Use as a catalyst - The main advantage in using ILs as
catalysis is that they are considered green catalysts.
• Chromatographic applications- ILs has shown
potential benefits in separation science15
• Solvent extraction - Some room temperature ILs are
used as extractors for separation of metal ions
• Sensing using ionic liquids –Chemo-sensing is studied
using ILs by observing the rapid decrease in viscosity.
• Spectroscopy - The use of ILs in mass spectroscopy is
rapidly increasing after being established by Armstrongʼs
group.
These are a few of the general uses of ILs. Other uses that
are related to oil and gas industry include:
• Desulfurization of fuels
• Extraction of naphthenic acids
• Denitrogenation of gasoline
• Use as demulsifiers
• Contaminants removal
• Selective gas separation and mercury removal in natural gas
• Biofuel synthesis or production
• Potential use in Enhanced Oil Recovery (EOR)
• CO2 Capturing and sequestration
• Use as wax and asphaltene inhibitors
• Extraction of heavy oil or bitumen with ILs
• Applications of Deep Eutectic solvents (DES)s in oil fields
Use of Ionic Liquids in the Petroleum Industry
Ionic Liquids for CO2 Capture
The global increase in energy demands due to increasing
population and industrialization are being met by fossil fuels
such as natural gas, coal, oil which make up 85% of energy
demand. Simultaneously, there is an exponential increase in
carbon dioxide emissions into the atmosphere from the
combustion of fossil fuels and therefore the need to reduce
anthropogenic emissions of CO2 the associated greenhouse
effect. Thus initiatives taken to capture CO2from natural gas
based plants are important not only to mitigate the effects of
global warming but also to be used as efficient injection fluid
for enhance oil recovery (EOR) in the petroleum reservoirs. CO2-EOR is a tertiary recovery method which has been used in the oil and gas industry for over 40 years. It has been
successfully implemented in oilfields in countries such as the
United States and in the North Sea in Norway. There is also a
growing interest in using CO2 for enhanced coal bed methane
production (ECBM) and also CO2 sequestration.
The first step in obtaining CO2 for the processes mentioned
above is to capture and separation. The three main processes
which are considered for the capture of CO2are,postcombustion, precombustion, oxyfuel combustion and natural gas sweetening
as well as amine-capturing technology. Some CO2 capture
processes are already being developed on a laboratory scale or
demonstrated in industrial pilot scale, that require various
processes involving physisorption/chemisorption, membrane
separation or molecular sieves, carbamation, amine dry scrubbing, mineral carbonation etc [3] [16]. Conventional technologies used
for CO2 capture during postcombustion process are solventbased
chemical absorbers such as aqueous amines. CO2 is
captured by chemical absorption process when flue gas streams
are passed through a chamber containing aqueous amines [17]. Some commonly used aqueous amines such as monoethanolamine (MEA), diethanolamine (DEA), triethanolamine (TEA), diglycolamine (DGA) and methyldiethanolamine (MDEA) are
used for CO2 capture through carbamate/carbonate formation. Amines are referred to as “conventional absorbers” because they
have been used for decades18.
The effectiveness of amines to capture CO2 may be attributed
to their properties such as high reactivity, high absorbing capacity (in terms of mass of CO2), CO2 selectivity and thermal stability
[19]. Nonetheless, some disadvantages that may be associated
with the use of amines for CO2 capture may be attributed to their
high vapor pressure, corrosive nature and high-energy
requirement for regeneration. The high vapor pressure of amines
causes them to be emitted into the atmosphere upon heating
and possibility of forming toxins such as nitrosamines due to their
unstable nature. Amines also take part in reactions which produce
waste products which are corrosive to process equipment. This is
especially true for MEA. During the regeneration/recycling
process, thereʼs a high requirement for energy in order to break
the chemical bonds formed between CO2 and amine. This causes
the amines to be degraded and affects their CO2 capture
efficiency. This makes them unrecyclable and need to be replaced
frequently [20] [21] [22]. Figure 3 shows a flowchart for Flue Gas
sweetening and CO2 capture Processes [23].
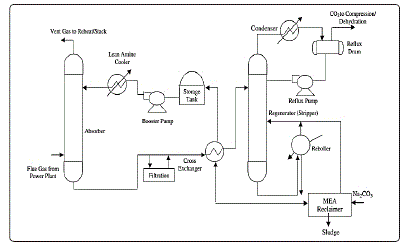
Figure 3: Flowchart for Flue Gas sweetening and CO2 capture Processes [23]
In recent times ILs have been considered for the capture of
CO2 due to their wide range of physical and chemical properties. They can be modified by their cationic and anionic moieties to
serve as efficient CO2 capture medium by absorption through
physical solubility and ionic interactions [23].
Kumar et al [12]. discussed some of these methods
elaborating their advantages and disadvantages [3] [24]. ILs
received attention for their potential as a CO2 and possibly
H2s absorber in the past few years for their ability to dissolve
these gasses at higher mole fractions [3] [25]. They also
possess physical and chemical properties which allow them to
be tailored for a specific application environment [3] [26] (negligible vapor pressure, high thermal stability and the
varieties of possible combinations between the anions and
cations to compose a tailor made IL are some of them). An
important requirement of the material used to separate CO2
from other component of flue gas is the selectivity property in
removing the CO2without affecting other components in the
gas mixture. Some of the ILs have such properties and thus
they are becoming attractive alternative for such uses [27].
The study of CO2 capturing using ILs was first conducted
by - Blanchardet. Al [28] which proved higher affinity to CO2
compared to amines.Following their study, many researchers
showed interest in studying these compounds for gas
processing in persuasion for a greener technology. Studies
have been conducted on the solubility of CO2 in ILs and also
to understand the phase behavior of IL-CO2 combination. Other studies focused on theoretical aspects such as the
interaction between CO2 and the anions present in the ILs. The
general findings are that solubility of CO2 is highly dependent
on the nature of the anionic component in the ILs rather than
the cationic nature of the molecule [26] [27].
Klahn and Seduraman [29] studied 10 different pure and
CO2-saturated ionic liquids through molecular dynamics
simulator based on empirical force field on liquid-phase charges. The partial molar volume of CO2 in ionic liquids (ILs) varies from
30 to 40cm3/mol. The study shows that the absorption of
CO2 does not affect the overall organization of ions in the ILs, neither solubility of CO2 in ILs is influenced by the direct CO2–ion
interactions. Instead, a strong correlation between the ratio of
unoccupied space in pure ILs and their ability to absorb CO2 is
found. Rather the preformed unoccupied space between the
ions is dispersed and expanded throughout the ILs accommodate
CO2 [29]. This phenomenon is explained in Figure 4.
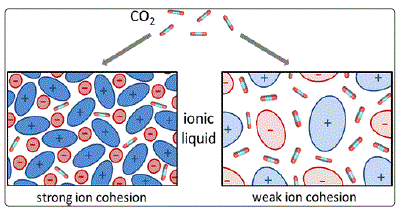
Figure 4: CO2 Solubility in Ionic liquids [29]
Kumar and Cho [12] have summarized a list of ILs used for both CO2 and H2s capturing process (Table 3). Experimental results on CO2 solubility in ILs with the experimental conditions and the type of IL used are also available in the public domain [3].

Table 3: List of ILs used by previous investigators for H2s and CO2 Capturing [12]
Several studies have shown the possible and efficient use of ILs as a mean for CO2capturing. However, their high cost is a disadvantage which led to the suggestion of pairing them with an amine. This led to IL-amine blends which have the advantages of both ILs and amine in achieving higher efficiency and reducing cost. Other advances in improving the efficiency of ILs for CO2 capturing are to emulsify the insoluble amines in a continuous phase of ILs. This provided a more cost effective solution [3] [30].
Ionic liquids as a substitute of surfactant for EOR
In an effort to meet the growing energy demand, tertiary
recovery or enhanced oil recovery methods are employed
after primary and secondary recovery of oil. Conventional oil
recovery such as primary recovery produces only about 5–10% of the total oil in the reservoir. Secondary recovery up to 40% or more by means of water and/or gas flood are recoded in
many oil fields. Chemical EOR methods involve the use of
chemical additives in flood water to improve its potential to
release trapped oil and push out from the reservoir rocks. Some of the common chemical flooding methods are surfactant flooding, polymer flooding, alkaline flooding, micellar flooding and Alkali-Surfactant-Polymer flooding (ASP). A properly selected surfactant solution has the ability to
lower the interfacial tension between water and oil from 10– 30 dynes/cm to the order of 10-3 dynes/cm, thus breaking up
the oil into micro-droplets so that they can be extracted from
the pores by hydrodynamic force of the chase water. They
also help in altering the wet ability of the reservoir rocks which
may be a favorable attribute in recovering additional oil. In
some cases co-surfactants (such as octanol) are used to
augment the surfactant flood process. In polymer flooding, polymers are added to injection water which enhances
viscosity of flood water thus ensuring the mobility ratio
between the displacing fluids and the displaced fluid below
unity which in turn improves volumetric sweep efficiency. In
alkaline flooding, high pH alkaline water is injected into the
reservoir which reacts with the acidic components of the
crude oil and form surfactants in-situ. Due to the large
quantities of surfactants required to displace meaningful
amounts of extra oil, the use of surfactants becomes
uneconomical in most situations. A solution to this drawback
of surfactant usage is the development of Alkaline-Surfactant-
Polymer (ASP) flooding. This type of flooding involves the
injection of a chemical formulation made up of an alkali with
some amount of surfactants into the reservoir. This reacts with
the crude oil to form micro-emulsion at the flood front, which
is later swept from the pores of the reservoir rock by high
viscous polymeric fluid.
In this section we will discuss the latest development on
new solvents and surfactant-IL combinations as an alternative
to conventional surfactant flooding process. The chemical
structure of the conventional surfactants are made up of a
polar hydrophilic part and often a long-chain, non-polar
hydrophobic part while in a surfactant-IL system, the surface
activity maybe associated to the anion, the cation or both biamphiphilic
or catanionic surfactants31. Some advantages of
ILs over traditional surfactants, which make them good
candidates to replace surfactants are that they are mostly
liquid and non-volatile, they can be specially designed for
specific tasks or reservoir conditions and they have a relatively
high viscosity which prevents them from phase-fingering
phenomenon due to unfavorable mobility ratio. They also do
not require co-surfactants since they have strong cohesive
forces which enable them to form stable micelles without the
need of additional chemicals [32].
Lago et al. [32] investigated tri-hexyl-(tetradecyl)-
phosphonium-chloride [P6 6 6 14] [Cl] ionic liquid for its ability to
act as a surfactant in reducing interfacial tension (IFT) between
crude oil and water at room temperature. The objective of the
study was to evaluate the possibility of ionic liquids to be used
for miscible EOR. The surface activity of [P6 6 6 14] [Cl] was
studied through measurements carried out to determine the
IFT, density, and viscosity of an equilibrium phase formed by
various mixtures of the IL [P6 6 6 14] [Cl], water and dodecane. They also studied the effect of salinity on the surface activity
of the IL by replacing the water with a brine solution (4% (w/w) NaCl).This is done since some reservoirs contain high salinity brine formation. For the binary system of water and
[P6 6 6 14] [Cl], the interfacial tension was reduced to1.8 mN/m. When [P6 6 6 14] [Cl] was added to a binary system of water and
dodecane to form a tri-phasic system, the interfacial tension
measure changed from 52.2 mN/m to about 1.4 mN/m. Even
though the interfacial tension obtained was not close to the
values obtained in conventional surfactants, the results
encouraged further investigations with other ILs and
combination of ILs. With regards to the replacement of water
with brine, it was observed that the ionic liquid in the mixture
shielded any effects of the salt. They also observed an increase
in the viscosity of the water phase when ionic liquids were
added. This may be a very useful property since it could
prevent the flood water from seeping through high
permeability regions or fractures and thus delay high water
cut production. This may also help to decrease the water-oil
mobility ratio within the reservoir which may lead to more
efficient oil recovery. Subsequently Lago et al [33]. Also
investigated the influence of temperature, on the phase
behavior, viscosity and interfacial tension of a mixture of
water, [P6 6 6 14] [Cl] and Dodecane (at atmospheric temperature
and at 75°C).The binary mixtures of [P6 6 6 14] [Cl] and dodecane
were found to be completely miscible at 75°C, while the same
binary system at 25°C was seen to be partially miscible. A
triphasic system was created between water, dodecane and
[P6 6 6 14] [Cl] and it was observed that there was little influence
of temperature on the equilibrium compositions 33. Hezave et
al [34]. conducted a study to observe how the IL 1-dodecyl-3-
methylimidazolium chloride [C12mim] [Cl] could reduce
interfacial tension(IFT)between water and crude oil. It was observed that there was a decrease in interfacial tension up
from 39.98 mN/mto 6.87 mN/m at 5000ppm concentration of
[C12mim] [Cl]. Surprisingly when the IFT was measured again
between formation brine and crude oil there was a significant
decrease in IFT with only 100 ppm of [C12mim] [Cl]. This
reduction in IFT with low concentrations of IL in salt water
compared to higher concentrations when IL in distilled water
is attributed to the absence of any ion either positive or
negative in the distilled water. Thus the IL molecules were
incapable of arranging themselves freely at the oil-water
interface because the high charge density of ILs lead to
molecular repulsion. However, in saline water the presence of
negative charges neutralize the positive surface charges of
the cationic part of IL. This resulted in an easier accumulation
of IL molecules at the oil-brine interface, and further reduction
in IFT [34]. The effect of NaCl concentrations on IFT of ILcrude
oil system was also investigated at different
concentrations of the IL [C12mim] [Cl]. It was observed that at
NaCl concentrations up to 100,000 ppm (far higher than
reservoir formation brine), the IL significantly reduces the IFT
to values lower than conventional surfactants.
The effects of temperature on the interfacial tension
between [C12mim] [Cl] and crude oil was also considered for
the study. The temperature was varied from 293.15K to 333.15
K. It was noted that the minimum temperature corresponding
to a minimum interfacial tension is the phase invasion
temperature (PIT). At temperatures below the PIT, there is a reduction in IFT, however at temperatures above the PIT, further increase in temperature resulted in an increase in the
IFT. This can be explained as being the result of the surfactant
or [C12mim] [Cl] adsorption onto the interface and diffusing
into the oil phase, resulting in emulsion inversion as the ionic
liquid content is accumulated to some extent [34] [35] [36].
Hezave et al [34]. Further looked into different families of
ionic liquids and their functionality in harsh reservoir
conditions such as high salinity and high temperature, at
which most surfactants lose their functionality. Success in this
direction would boost chemical EOR applications in the
reservoirs where presently no solution is available.
Interfacial tension (IFT) measurements were conducted
between crude oil and four different types of IL solutions
namely [C12mim] [Cl], [C8mim] [Cl], [C12 Py] [Cl] and [C8Py] [Cl]
using the pendant drop and spinning drop techniques to
measure the effects of NaCl concentrations, temperature and
absence/presence of ions. Their results showed that all the
four ILs were more effective in the presence of salinity in
reducing IFT as compared to conventional surfactants. The
functionality of the ILs are seen to diminish as the temperature
increased, and this was attributed to the presence of nitrogen
atoms in the imidazolium and pyridinium based ILs. It was
concluded that the ILs exhibited good properties in reducing
IFT in harsh reservoir salinity conditions. However, before
implementing these ILs to a field scale, further studies should
be conducted to design an IL solvent which can also withstand
high reservoir temperatures and also their adsorption on
reservoir rocks should be studied. One of the main drawback
of surfactants is their high adsorption on the reservoir rocks
which leads to a change in rock wettability towards more
water wet conditions thus favoring release of oil and improved
recovery. However this property has negative impact on the
economics of EOR. Therefore these new ILs as a substitute of
surfactant flooding should be designed to optimize the
adsorption on the reservoir rocks at the same time keeping in
mind the economics of the project.
Further studies on the over, the effects of [BMIM] [ClO4]
on the interfacial tension of oil− water and oil recovery are
tested. Results shows that [BMIM][ClO4] can improve the
mobility of heavy oil by reducing the IFT of oil−water and
increase the recovery factor by 79.94% [37].
Shaktivel et al [38]. performed similar experiments using
ionic liquids with imidazolium cation and various anions to
study how these ionic liquids could lower the interfacial
tension in crude oil-water systems and the synergic effect of
ionic liquids and NaCl in lowering the IFT of crude oil-water
systems. The Wilhelmy plate method was used to study the
surface tension and interfacial tension of aqueous solutions of
the ionic liquids and crude oil systems, with and without
salinity effect while considering the concentration of the ILs
and also the effect of temperature on the system. The ionic
liquids synthesized and used in this experiment are 1-butyl-3-
methylimadazolium chloride, 1-butyl-3-methylimadazolium
bromide,1-butyl-3-methylimadazolium tetra-fluoroborate, 1-butyl-3-methylimadazolium dihydrogen phosphate, 1-butyl-3-methylimadazolium hydrogen sulfate, 1-butyl-3-
methyl-imadazolium hexa-fluorophosphate, 1-hexyl-3-
methylimadazolium bromide, 1-hexyl-3-methyl-imadazolium
hydrogen sulfate and 1-octyl-3-methylimadazolium chloride.
The interfacial tension of the crude oil-water systems is
greatly dependent on the adsorption of IL molecules at the
interface between crude oil and water. In this study, it was
observed that the concentration of ILs also affects the
interfacial tension between crude oil and water [39]. The IFT
measurements at 288.15 K between crude oil and water, with
concentration change from 0 to 50 ppm, was observed to
decrease from 36.24 to 25.86 mN/m for IL [C4mim] [Cl] and
for [C8mim][Cl] it was reduced from 36.24 to 23.26 mN/m. It
can be inferred from the rest of the experiments that, the
longer the alkyl chain length, the more effective the IL is at
reducing the IFT at lower concentrations. The IFT of crude oilwater
systems were also measured in the presence of ionic
liquids in presence of NaCl. The synergetic effect of NaCl and
ionic liquids was observed as the IFT was reduced from 21.37
to 9.26 mN/m, where as only IL at a concentration of 1000
ppm the IFT was reduced from 21.20 to 19.58 mN/m [39] [40].
Aside from focusing on the reduction of IFT some
researchers also observed the changes in rock wettability and
relative permeabilities. Dahbag et al [41]. conducted studies
on different IL solutions to observe how they change the
wettability of a reservoir rock. Initially, they screened different
ILs based on their solubility in different brine compositions, thermal stability and IFT reduction in high salinity and hightemperature
conditions. After selecting the most suitable
ionic liquid, they went on to conduct several core flooding
experiments at reservoir conditions using Berea sandstone
cores to investigate the ability of the ILs to adsorb on rock
surfaces and change their wettability. After the initial screening
of nine ionic liquids, tetra-alkyl-ammonium sulfate was found
to be the most potent ionic liquid. IFT measurements of tetraalkyl-
ammonium sulfate with crude oil at different IL
concentrations and high solution salinity showed a reduction
in IFT even with increasing levels of salinity. Temperature and
pressure had little effect on these measurements. The
tendency for adsorption on rock surfaces at reservoir
conditions was observed and even in high salinity ionic
solution. After core flooding experiments, wettability changes
were confirmed by performing contact angle measurements. Wettability changed from slightly oil wet to mediumwaterwet
condition as the IL concentration was increased [41].
In most research works, the imidazolium family of ILs with
chloride counter-ions are given maximum attention. In some
studies counter-ions and /or pyridinium, ammonium and
phosphonium cations are used. In a study by Rodriguez-
Palmeiro et al [31]. A surfactant-IL comprising of 1-dodecyl-3-
methylimidazolium cation and acetate anion [C12 mim] [OAc]
was synthesized and used for a series of dynamic interfacial
tension studies in varying temperature, water salinity and
some alkaline additives. The results obtained with the new
surfactant ionic liquid were compared with the results found
from the literature of similar ionic liquid but with different counter-ions which were halides (chloride, bromide, and
iodide).The effects of aggregation of [C12mim] [OAc] was
investigated and it was seen to exhibit a lower critical micelle
concentration and a better tendency for micellization over
adsorption at the interface and lower spontaneity for
micellization [31].
Promising results were also obtained from dynamic
interfacial tensions of aqueous solutions of [C12mim] [OAc]
with crude oil various compositions. From the results, there
was a lowering of the interfacial tension and higher stability in
presence of salts (up to 4%wt NaCl). In presence of alkalis, the
interfacial tension was further reduced. In mostliterature
where surfactant ionic liquids were used, interfacial tension
values were reduced toas low as 1mN/m. Results obtained
from Rodriguez-Palmeiro et al. [31], show a lower interfacial
tension of at least one order in magnitude, with larger effects
being observed when strong alkalis like NaOH are used.
Heavy Oil dissolution and Viscosity reduction
One of the challenges of the upstream petroleum industry
is the recovery of heavy and extra heavy crude oil, which is
estimated to be more than double the volumes of conventional
light to medium crude oils discovered throughout the globe. The heavy fraction (wax, asphaltenes and resinous compounds) present with the crude oil are responsible for the higher
viscosity and density of the crude oil which poses challenges
not only in production but also in surface processing and
refining [42]. Because easily extractable lighter oil reserve is
steadily decreasing, the industry is becoming rapidly
dependent on economic extraction of the heavy and extraheavy
oil that was previously considered uneconomical.
For the purpose of heavy oils recovery, thermal methods (steam injection and in-situ combustion) are the most
successful methods, while non-thermal methods such as
miscible gas injection and solvent injection found limited
success in real field scenario. The problems of transporting
heavy and viscous crude oils through long distance pipelines
and assuring its flow is another great challenge that sometimes
make the project un-economic. Since the resistance to flow is
originated form the oil microstructure, partial upgrading
through the modification of the microstructure by heating
and solvent dilution have been the most widely applied
methods in pipeline transportation of heavy oils. Dilution of
heavy and extra heavy oil is achieved through the addition of
less viscous crude oils, condensate, gasoline, kerosene or
naphtha [43] of which light hydrocarbons and condensates (C5+) are the most widely used diluents [44]. However the
high price of the diluents, difficulties in separation and
recycling, availability of light oils close to heavy oil fields, and
the environmental and climate specific issues put serious
restrains on heavy oil dilution process. There is also the issue
of compatibility between the crude and the solvents which
may results in solids precipitation and plugging. This
necessitates increasing solvent polarity [45] or use of aromatic
solvents to prevent asphaltene precipitation and the resulting
flowline blockage [44], thus increasing operational cost as
well as the environmental concerns. These constraints alongwith data from early works encouraged studies on ionic liquids
as a replacement/aid for solvent dilution which is the topic of
discussion of the flowing section.
One of the initial works on heavy oil upgradation was
tried with 1-butyl-3-methylimidazolium chloride [BMIM]
[AlCl4] ionic liquid reported by Fan et al. [46]. More than 60% of oil viscosity could be reduced by treating with [BMIM]
[AlCl4] in presence of transition metal salts (Fe & Ni). The
process was seen to be more efficient viscosity reducer when
certain amount of sulfur was present in the oils and the water
content in the oil was less than 10%. Further progress on the
similar class of ILs, the modified versions of 1-butyl-3-
methylimidazolium tetrachloroferrate [BMIM] [FeCl4], were
prepared by Shaban et al [47]. and studied through
physicochemical methods and catalytic activity measurements, with special emphasis on the reaction temperature and the
water content in the heavy crude oils. It was found that [BMIM]
[FeCl4] family of ILs have the best effect on the heavy crude oil
upgradation between 70–90°C temperature and water
content less than 8% [47].
Saaidet al [37]. further investigated on a different class of
BMIM ILs. They synthesized1-butyl-3-methylimidazolium
perchlorate, [BMIM] [ClO4], and studied their effect on
viscosity, density, SARA (saturates, aromatics, resins and
asphaltens) contents, elemental compositions and molecular
weight. The results indicated that the presence of [BMIM]
[ClO4] significantly changes the composition of the heavy oil
which resulted in reduction of viscosity and density of the
crude and improved flow ability.
Sakthivel et al [38]. Investigated a set of eight different
ionic liquids along with five solvents, namely heptane, toluene, decane, ethyl acetate, and hexane to assess the possible
dissolution effect on heavy crude oils. The ionic liquids used in
this study were: diethylammonium phosphate
[Et2NH2]+[H2PO4]-, diethylammoniumsulphate [Et2NH2]+[HSO4]-, triethylammonium acetate [Et3NH]+[CH3COO]-, triethylammoniumtetrafluoroborate [Et3NH]+[BF4]-, triethylammoniumsulphate [Et3NH]+[HSO4]-
,tripropylammoniumsulphate [Pr3NH]+[HSO4]- and
tributylammoniumsulphate [Bu33NH]+[HSO4]-. Usually the
treatment of heavier hydrocarbons such as asphaltene is done
by aromatic solvents such as benzene, xylene, and toluene. Even though these aromatic solvents are efficient asphaltene
dissolvers, they are however volatile and hazardous to human. In these experiments about 10% of ionic liquids were dissolved
in organic solvents and their dissolution effect on the heavy
crude oil studied. It was observed the [Et2NH2]+[H2PO4]-
exhibited good performance in the dissolution of heavy oil in
the presence of toluene. [Et3NH]+[CH3COO]- performed better
in heptane, decane, and hexane, and most interestingly in
ethyl acetate solvent the [Et2NH2]+[H2PO4]-IL exhibited much
improved results. The efficiency of the dissolution of heavy oil
by solvents was in the following order; toluene > heptane
>decane> ethyl acetate > hexane. This work shows that with
minimal addition of ionic liquids to heavy crude oil the
dissolution effect is markedly enhanced. Further research on the similar path were conducted using only ionic liquids
without any solvents to identify the effect of only ionic liquids
on the dissolution of heavy crude oil and asphaltenes in heavy
crude oils [2] [38] [48] however the results are not so promising.
In continuation to the previously mentioned studies, Sakthivelet al [38] [49]. conducted further research on the use
of ionic liquids to dissolve and reduce the viscosity of
tankbottomsludge (TBS) with ILshaving imidazolium cation
[BMIM]+ and various anions such [Cl]-, [Br]-, [BF4]-, [H2PO4]-, [HSO4]- and [PF6]-. UV-visible spectrophotometric techniques
were used to observe absorbance intensity with respect to a
particular ionic liquid for a concentration range from 10 to 70
ppm. IL content of 10 ppm was found to be adequate for
nearly complete dissolution. They also carried out dissolution
studies of the tankbottom sludge in the presence of ionic
liquids in different solvents such as toluene, heptane, decane, hexane and ethyl acetate and found that [BMIM]+[PF6]- gave
better dissolution performance in toluene and hexane, while
in heptane [HMIM]+[Br]- performed better. [BMIM]+[H2PO4]-
performed better in the presence of decane and
[BMIM]+[H2PO4]- and [BMIM]+[Br]- gave better dissolution of
TBS in the presence of ethyl acetate irrespective of the weight
ratio of TBS:ILs [49]. Another explanation for the effective
dissolution of tankbottom sludge containing ionic liquids
could be due to the fact that there is an interaction between
the ionic liquids and the asphaltenes which eventually breaks
the asphaltene macro-structure. This is because there is an
interaction between the cationic part of the ionic liquid and
the heteroatomic functional groups of asphaltenes (which is
the major constituents of the TBS) such as Sulphur, Oxygen, and Nitrogen. The heteroatoms of the asphaltenes contain at
least one lone pair of electrons which are available for the
ionic interaction. At higher concentrations of the ionic liquids, the asphaltene molecules are effectively surrounded by ILs
and solvated by the interaction forces. Arresting of the
heteroatom activity in the asphaltene/resin moiety leaves the asphaltene/resins moiety with only hydrocarbons which
dissolve easily in the organic solvent [50]. Further studies on
the interactions between asphaltenes and ionic liquids can
lead to the development of an environmentally friendly
inhibitor to mitigate flow assurance issues in the oilfield.
Synergistic effect of ionic liquids along with brine has also
been studied. It is observed that, solubility of heavy crude oil
in the presence of ionic liquids and the solvents increases
about 60 % when water content is minimum. Reduction of
interfacial tension is more effective in presence of salt in the
mixture. The above findings reveal that it may be possible to
minimize the amount of organic solvents that may be required
to upgrade heavy oil and tank bottom sludge and may also
help in surface and subsurface flow-assurance issues by
employing suitable IL-Solvent combinations.
Ionic Liquids for Asphaltene Inhibition
As discussed above the precipitation and deposition of
heavy organics in crude oil like wax and asphaltenes poses
great challenge during crude oil production, transportation and storage, of which the concern for asphaltene deposition
is significantly higher. Agglomeration and deposition of
asphaltenes can be caused due to change in pressure, temperature, pH and composition of crude oil [51]. These
depositions can occur in the near wellbore reservoir, subsurface
production tubing, surface flowlines and oil processing
facilities, which often leads to a decline in production of oil
and/or complete shut-in of the well, resulting in loss of
production and additional operating cost for remediation and
cleaning operations [52]. Waxes are long chain paraffin
components of crude oil and rather easy to handle, mostly by
heat management, which is not the case for asphaltene. Asphaltenes are extremely complex molecules, defined as
“the heaviest components of crude oil which are insoluble in
lower alkanes (n-pentane or n-heptane) but soluble in
aromatic solvents such as benzene, toluene, and xylene. The
chemical structure of asphaltenes is uncertain due to their
complex and uncertain nature [53] [54] [54]. When asphaltenes
precipitate and deposit in the reservoir, they may cause
permeability and porosity reduction and also wettability
alterations from water–wet to oil-wet [56], an unwanted
phenomenon which results in lowering oil relative permeability
flow efficiency. It is observed that reservoirs which undergo
enhanced oil recovery (EOR) processes like hydrocarbon gas
or miscible CO2 injection are faced with severe asphaltene
problems regardless of crude oil density and viscosity. Therefore prior to implementing any enhanced oil recovery
project, it is recommended that a careful study of the
probability of asphaltene precipitation and deposition be
carried out in order to consider the preventive measures or
mitigation strategies [54] [57] [58].
In the likelihood of asphaltene precipitation, chemical
treatment techniques such as asphaltene inhibitors are
employed to prevent the aggregation of asphaltene molecules
and to enhance their stability in the crude oil. In most cases
however, the solvent treatments are carried out to dissolve
already precipitated asphaltene in the wellbore and surface
flow lines. Some conventional asphaltene dissolvers are
toluene, xylene, and benzene, however, these chemicals are
flammable, carcinogenic, dangerous to handle and harmful to
the environment [59].
The next generation asphaltene inhibitors are based on
their surfactant characteristics and the most prominent
among them is the dodecylbenzenesulphonic acid (DBSA). These surfactants must have the ability to stabilize the
suspended asphaltene colloids and also dissolve asphaltenes
in the molecular level through acid-base interactions [40] [50]
[60] [61] [62]. Recently, ILs have attracted attention in the area
of asphaltene inhibition due to their effectiveness in
dissolution of heavy crude oil, and also the ability to disperse
asphaltenes in crude oils [60][63] [64] [65].
One of the early investigations performed by Hu and Guo
[63] was on the effect of ionic liquids and amphiphiles on the
inhibition of asphaltenes precipitated from CO2 injected oil
reservoirs, where miscibility between oil and CO2 occurred. Ionic liquids with different types of cationic groups and anions were investigated, with emphasis on the effect of the cation
tail lengths, head groups, cation and anion combination and
the concentration of the ionic liquids. The studies were
performed on oil with CO2in miscible condition (above
minimum miscible pressure) and the asphaltene precipitation
was measured quantitatively. The study revealed that the
ionic liquids with cation being p-alkylpyridinium and with a
chloride anion [Cnpy] [Cl] showed effective inhibition with
decreasing alkyl chain length, in the following order: [C12py]
[Cl] < [C8py] [Cl]< [C4py] [Cl]. The shortest chain cation
performed better than longer chain length cations. While
comparing ionic liquids of different cations but the same
anion, [C4iql] [Cl] was observed to be more effective than
[C4py] [Cl] in inhibiting CO2 instigated asphaltene precipitation
[63].
Direct coal liquefaction (DCL) is a source of various
industrial chemicals and liquid fuels. Asphaltenes, which
constitute about 25 wt. % of the DCL product,is a rich source
for aromatic chemical precursors, which needs to be extracted
from the DCL product prior to shipment. Bai et al [64]. developed a series of Protic ionic liquids (PIL) using N-methylimidazole, 3-methylpyridine and triethylamine
cations combined with formate, acetate, propionate and
benzoate anions to extract asphaltenes from DCL product at
room temperature showing higher yields compared to
conventional solvents. The extracted asphaltenes have lower
H/C ratios, higher degree of aromaticities, lower sulfur
contents, no ash contents and nearly no quinoline insolubles. Increasing alkyl chain length of anions enhanced extraction
yields whereas extraction yields of asphaltenes varied with
different cations of PILs in accordance with the order of
N-methylimidazolium[MIM]+< triethylammonium[TEtA]+< 3-methylpyridinium [MPy]+. Hydrogen bonding, π–cation
interactions, and charge-transfer between complexes are
attributed as the responsible functions for asphaltene
dissolution [64]. Junaki et al [65]. prepared IRAN91, an IL
prepared from mixing of AlCl3 with [Et3N] HCl for upgradation
of heavy oil and asphaltene stabilization purposes. The
compound is seen to reduce the asphaltene content, viscosity, and average molecular weight of the heavy oil significantly. Viscosity is reduced from 1800 to 644 cP, Average molecular
weight came down from 2840 to 384 and the asphaltene
precipitation is reduced from 15 to 7%. The important
phenomena observed in this process is the formation of a
complex between the IL and the organic sulfurin heavy oil, which weakened the C-S bonds resulting in the drop of average
molecular weight and higher stability of asphaltene in the oil
[65].
Adeniji et al [66]. carried out a series of experiments to
study the properties of the imidazolium IL cation with various
anions targeting asphaltene dispersion. The ILs synthesized
and used in these studies were 1-butyl-3-methylimidazolium
chloride, 1-butyl-3-methylimidazolium nitrate and 1-methyl-
1H-imidazol-3-ium-2-carboxy-benzoate. Through
computational simulations [66] they inferred that the
interactions between the ionic liquids and the asphaltenes occurred through the π-π interaction between cation and
asphaltenes via hydrogen bonding, similar to the observations
made by Bai et al. [64]. Based on the interaction energies, the
order of reactivity between the asphaltenes and ionic liquids
were deduced. The interaction energies of 1-butyl-3-
methylimidazolium chloride, 1-butyl-3-methylimidazolium
nitrate and 1-methyl-1H-imidazol-3-ium-2-carboxybenzoate
were -55.4×104,-44.1×104 and -54.8×104 kcal/mol, respectively. From the interaction energies, it was inferred that 1-butyl-3-
methylimidazolium chloride presented the smallest interaction
energies and was a better candidate in the dispersion of
asphaltenes.
From the above results and several other similar studies (which could not be discussed for space constraints) it can be
inferred that ionic liquids have good potential in keeping the
asphaltene in solution and contain the oilfield flow assurance
problem in an effective and environment friendly manner.
Summary and Conclusion
This review article discussed the available literature
information about the applications of ionic liquids in the
petroleum industry. More effort has been given to the
upstream applications of ILs as there are several reviews
availble on the downstream applications of ILs, which is not
the case for upstream application of ILs.
Many ILs are proven to be promising in CO2 capture and
imidazolium-based ILs are found to be most promising in
reducing IFT even at high temperature and high salinity
environments which make them suitable for high temperature
EOR applications. This paved ways for further researches in
order to develop ionic liquids with improved properties which
could replace surfactants currently used for chemical
enhanced oil recoveries, particularly in high temperature and
high salinity reservoirs which are not suitable for surfactant
application. It is also seen that ILs can find potential
applications in heavy oil recovery, upgradation for pipeline
flow and controlling sludge precipitation in storage tanks. Asphaltene inhibition and better flow assurance particularly in
miscible gas EOR project is another potential area where ILs
can be used with case specific studies.
At present the major drawback of applying ionic liquid in
large quantity is their cost. As much as they are green solvents, which makes them potential substitute for volatile organic
solvents, they are quite expensive, which so far has restricted
their use in the oil and gas industry. Even though some
literature suggests the method of recycling and reuse, rapid
applications of ionic liquids in the petroleum industry will not
be anytime soon, due to their cost. However, if cost effective
means are found to synthesize them on a large scale, they
could be widely used to replace many potentially harmful and
volatile organic solvents and chemicals currently used in the
oil industry.
Acknowledgement
The authors sincerely acknowledge the material and financial support of the Petroleum Institute, Abu Dhabi, UAE.
References
- Rogers RD, Seddon KR. Ionic liquids: industrial applications for green chemistry. ACS Publications. 2002; ISBN-13: 978-0841237896.
- Welton T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chemical reviews. 1999; 99(8): 2071-84. doi: 10.1021/cr980032t
- Karadas F, Atilhan M, Aparicio S. Review on the use of ionic liquids (ILs) as alternative fluids for CO2 capture and natural gas sweetening. Energy & Fuels. 2010; 24(11): 5817-28. doi: 10.1021/ef1011337
- Lunagariya J, Dhar A, Vekariya RL. Efficient esterification of n-butanol with acetic acid catalyzed by the Brönsted acidic ionic liquids: influence of acidity. RSC Advances. 2017; 7(9): 5412-20. doi: 10.1039/C6RA26722J
- Cole AC, Jensen JL, Ntai I, et al. Novel Brønsted acidic ionic liquids and their use as dual solvent- catalysts. Amer Chem Soc J. 2002; 124(21): 5962-63. doi: 10.1021/ja026290w
- Karimi-Maleh H, Rostami S, Gupta VK, Fouladgar M. Evaluation of ZnO nanoparticle ionic liquid composite as a voltammetric sensing of isoprenaline in the presence of aspirin for liquid phase determination. J Mol Liq. 2015; 201: 102-7. doi: 10.1016/j.molliq.2014.10.042
- Kim Y, Choi W, Jang J, Yoo KP, Lee C. Solubility measurement and prediction of carbon dioxide in ionic liquids. Fluid Phase Equilibria. 2005; 228-229: 439-45. doi: 10.1016/j.fluid.2004.09.006
- Rogers RD, Seddon KR. Ionic liquids--solvents of the future?. Science. 2003; 302(5646): 792-93. doi: 10.1126/science.1090313
- Alboudwarej H, Akbarzadeh K, Beck J, Svrcek WY, Yarranton HW. Regular solution model for asphaltene precipitation from bitumens and solvents. AIChE J. 2003; 49(11): 2948-56. doi: 10.1002/aic.690491124
- Pastre JC, Génisson Y, Saffon N, Dandurand J, Correia CR. Synthesis of novel room temperature chiral ionic liquids: application as reaction media for the heck arylation of aza-endocyclic acrylates. J Bra Chem Soc. 2010; 21(5): 821-36. doi: 10.1590/S0103-50532010000500009
- Plechkova NV, Seddon KR. Applications of ionic liquids in the chemical industry. Chemical Society Reviews. 2008; 37(1): 123-50.
- Kumar S, Cho JH, Moon I. Ionic liquid-amine blends and CO2 BOLs: prospective solvents for natural gas sweetening and CO2 capture technology-a review. Int J greenhouse gas control. 2014; 20: 87-116.
- Shiflett MB, Drew DW, Cantini RA, Yokozeki A. Carbon dioxide capture using ionic liquid 1-butyl-3-methylimidazolium acetate. Energy & Fuels. 2010; 24(10): 5781-89. doi: 10.1021/ef100868a
- Zhang S, Sun N, He X, Lu X, Zhang X. Physical properties of ionic liquids: database and evaluation. Journal of physical and chemical reference data. 2006; 35(4): 1475-1517. doi: 10.1063/1.2204959
- Pandey S. Analytical applications of room-temperature ionic liquids: a review of recent efforts. Analytica Chimica Acta. 2006; 556(1): 38-45.
- Hasib-ur-Rahman M, Siaj M, Larachi F. Ionic liquids for CO2 capture— development and progress. Chemical Engineering and Processing: Process Intensification. 2010; 49(4): 313-22. doi: 10.1016/j.cep.2010.03.008
- Puxty G, Rowland R, Allport A. Carbon dioxide postcombustion capture: a novel screening study of the carbon dioxide absorption performance of 76 amines. Environmental science & technology. 2009; 43(16): 6427- 33. doi: 10.1021/es901376a
- Torralba-Calleja E, Skinner J, Gutiérrez-Tauste D. CO2 capture in ionic liquids: a review of solubilities and experimental methods. Journal of Chemistry. 2013; 2013: 1-16. doi: 10.1155/2013/473584
- Samanta A, Zhao A, Shimizu GK, Sarkar P, Gupta R. Post-combustion CO2 capture using solid sorbents: a review. Industrial & Engineering Chemistry Research. 2011; 51(4): 1438-63. doi: 10.1021/ie200686q
- Riemer PW, Ormerod WG. International perspectives and the results of carbon dioxide capture disposal and utilisation studies. Energy Conversion and Management. 1995; 36(6-9): 813-18. doi: 10.1016/0196- 8904(95)00128-Z
- Karl M, Wright RF, Berglen TF, Denby B. Worst case scenario study to assess the environmental impact of amine emissions from a CO2 capture plant. International Journal of Greenhouse Gas Control. 2011; 5(3): 439- 47. doi: 10.1016/j.ijggc.2010.11.001
- Rao AB, Rubin ES. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environmental science & technology. 2002; 36(20): 4467- 475. doi: 10.1021/es0158861
- Olajire AA. CO2 capture and separation technologies for end-of-pipe applications–a review. Energy. 2010; 35(6): 2610-628. doi: 10.1016/j. energy.2010.02.030
- Boot-Handford ME, Abanades JC, Anthony EJ, et al. Carbon capture and storage update. Energy & Environmental Science. 2014; 7(1): 130-189.
- Abanades JC, Rubin ES, Anthony EJ. Sorbent cost and performance in CO2 capture systems. Industrial & Engineering Chemistry Research. 2004; 43(13): 3462-466. doi: 10.1021/ie049962v
- Anthony JL, Aki SN, Maginn EJ, Brennecke JF. Feasibility of using ionic liquids for carbon dioxide capture. International journal of environmental technology and management. 2004; 4(1-2): 105-115. doi: 10.1504/ IJETM.2004.004624
- Brennecke JF, Gurkan BE. Ionic liquids for CO2 capture and emission reduction. The Journal of Physical Chemistry Letters. 2010; 1(24): 3459- 464. doi: 10.1021/jz1014828
- Blanchard LA, Hancu D, Beckman EJ, Brennecke JF. Green processing using ionic liquids and CO2. Nature. 1999; 399 (6731): 28-29. doi: 10.1038/19887
- Klahn M, Seduraman A. What determines CO2 solubility in ionic liquids? A molecular simulation study. The Journal of Physical Chemistry B. 2015; 119(31): 10066-10078. doi: 10.1021/acs.jpcb.5b03674
- Ramdin M, De Loos TW, Vlugt TJ. State-of-the-art of CO2 capture with ionic liquids. Industrial & Engineering Chemistry Research. 2012; 51(24): 8149-177. doi: 10.1021/ie3003705
- Rodríguez-Palmeiro I, Rodríguez-Escontrela I, Rodríguez O, Arce A, Soto A. Characterization and interfacial properties of the surfactant ionic liquid 1-dodecyl-3-methyl imidazolium acetate for enhanced oil recovery. RSC Advances. 2015; 5(47): 37392-7398. doi: 10.1039/C5RA05247E
- Lago S, Rodríguez H, Khoshkbarchi MK, Soto A, Arce A. Enhanced oil recovery using the ionic liquid trihexyl (tetradecyl) phosphonium chloride: phase behaviour and properties. RSC Advances. 2012; 2(25): 9392-397. doi: 10.1039/C2RA21698A
- Lago S, Francisco M, Arce A, Soto A. Enhanced oil recovery with the ionic liquid trihexyl (tetradecyl) phosphonium chloride: a phase equilibria study at 75° C. Energy & Fuels. 2013; 27(10): 5806-810. doi: 10.1021/ef401144z
- Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B. Investigating the effect of ionic liquid (1-dodecyl-3-methylimidazolium chloride ([C12MIM][Cl])) on the water/oil interfacial tension as a novel surfactant. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2013; 421: 63-71. doi: 10.1016/j.colsurfa.2012.12.008
- Aveyard R, Binks B, Chen J, et al. Surface and colloid chemistry of systems containing pure sugar surfactant. Langmuir. 1998; 14(17): 4699-709. doi: 10.1021/la980519x
- Ye Z, Zhang F, Han L, Luo P, Yang J, Chen H. The effect of temperature on the interfacial tension between crude oil and gemini surfactant solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2008; 322(1): 138-41. doi: 10.1016/j.colsurfa.2008.02.043
- Md. Saaid I, Mahat SQA, Lal B, Mutalib MIA, Sabil MK. Experimental investigation on the effectiveness of 1-butyl-3-methylimidazolium perchlorate ionic liquid as a reducing agent for heavy oil upgrading. Industrial & Engineering Chemistry Research. 2014; 53(19): 8279-284. doi: 10.1021/ie500499j
- Sakthivel S, Velusamy S, Gardas RL, Sangwai JS. Experimental investigation on the effect of aliphatic ionic liquids on the solubility of heavy crude oil using UV–visible, Fourier transform-infrared, and 13C NMR spectroscopy. Energy & Fuels. 2014; 28(9): 6151-162. doi: 10.1021/ef501086v
- Sakthivel S, Velusamy S, Gardas RL, Sangwai JS. Use of aromatic ionic liquids in the reduction of surface phenomena of crude oil–water system and their synergism with brine. Industrial & Engineering Chemistry Research. 2015; 54(3): 968-78. doi: 10.1021/ie504331k
- Hashmi SM, Firoozabadi A. Self-assembly of resins and asphaltenes facilitates asphaltene dissolution by an organic acid. Journal of colloid and interface science. 2013; 394: 115-23. doi: 10.1016/j.jcis.2012.11.069
- Dahbag MB, Al-Quraishi A, Benzagouta M. Efficiency of ionic liquids for chemical enhanced oil recovery. Journal of Petroleum Exploration and Production Technology. 2015; 5(4): 353-61. doi: 10.1007/s13202-014- 0147-5
- Santos R, Loh W, Bannwart A, Trevisan O. An overview of heavy oil properties and its recovery and transportation methods. Brazilian Journal of Chemical Engineering. 2014; 31(3): 571-90. doi: 10.1590/0104- 6632.20140313s00001853
- Nunez GA, Rivas H, Joseph D. Drive to produce heavy crude prompts variety of transportation methods. Oil and Gas Journal. 1998; 96(43): 59- 68.
- Saniere A, Henaut I, Argillier J. Pipeline transportation of heavy oils, a strategic, economic and technological challenge. Oil & Gas Science and Technology. 2004; 59(5): 455-66. doi: 10.2516/ogst:2004031
- Gateau P, Henaut I, Barre L, Argillier J. Heavy oil dilution. Oil & gas science and technology. 2004; 59(5): 503-09.
- Fan Z, Wang T, He Y. Upgrading and viscosity reducing of heavy oils by [BMIM][AlCl4] ionic liquid. Journal of Fuel Chemistry and Technology. 2009; 37(6): 690-93. doi: 10.1016/S1872-5813(10)60015-1
- Shaban S, Dessouky S, Badawi AEF, El Sabagh A, Zahran A, Mousa M. Upgrading and viscosity reduction of heavy oil by catalytic ionic liquid. Energy & Fuels. 2014; 28(10): 6545-553. doi: 10.1021/ef500993d
- Fan H, Li Z, Liang T. Experimental study on using ionic liquids to upgrade heavy oil. Journal of Fuel Chemistry and Technology. 2007; 35(1): 32-35. doi: 10.1016/S1872-5813(07)60009-7
- Sakthivel S, Velusamy S, Gardas RL, Sangwai JS. Eco-efficient and green method for the enhanced dissolution of aromatic crude oil sludge using ionic liquids. RSC Advances. 2014; 4(59): 31007-1018.
- Hashmi SM, Zhong KX, Firoozabadi A. Acid–base chemistry enables reversible colloid-to-solution transition of asphaltenes in non-polar systems. Soft Matter. 2012; 8(33): 8778-785. doi: 10.1039/C2SM26003D
- Khanifar A, Sheykh AS, Demiral B, Darman NB. Study of Asphaltene Precipitation and Deposition Phenomenon during WAG Application. Paper presented at: SPE Enhanced Oil Recovery Conference. SPE- 143488-MS. 2011.
- Buenrostro-Gonzalez E, Lira-Galeana C, Gil-Villegas A, Wu J. Asphaltene precipitation in crude oils: Theory and experiments. AIChE Journal. 2004; 50(10): 2552-570. doi: 10.1002/aic.10243
- Mullins OC. The asphaltenes. Annual Review of Analytical Chemistry. 2011; 4: 393-418.
- Mullins OC, Sheu EY, Hammami A, Marshall AG. Asphaltenes, heavy oils, and petroleomics. Springer Science & Business Media; 2007.
- Speight JG. The chemistry and technology of petroleum. CRC press; 2014.
- Chrisman E, Menechini P, Lima V. Asphaltenes-Problems and Solutions in E&P of Brazilian Crude Oils. INTECH Open Access Publisher; 2012.
- Khaleel A, Abutaqiya M, Tavakkoli M, Melendez-Alvarez AA, Vargas FM. On the Prediction, Prevention and Remediation of Asphaltene Deposition. Paper presented at: Abu Dhabi International Petroleum Exhibition and Conference. SPE-177941-MS. 2015.
- Subirana M, Sheu EY. Asphaltenes: fundamentals and applications. Springer Science & Business Media; 2013.
- Junior LCR, Ferreira MS, Da Silva Ramos AC. Inhibition of asphaltene precipitation in Brazilian crude oils using new oil soluble amphiphiles. J. Pet. Sci. Eng. 2006; 51(1): 26-36. doi: 10.1016/j.petrol.2005.11.006
- Goual L, Firoozabadi A. Effect of resins and DBSA on asphaltene precipitation from petroleum fluids. AIChE journal. 2004; 50(2): 470-479. doi: 10.1002/aic.10041
- Hashmi SM, Firoozabadi A. Effect of dispersant on asphaltene suspension dynamics: Aggregation and sedimentation. The Journal of Physical Chemistry B. 2010; 114(48): 15780-5788. doi: 10.1021/jp107548j
- Hashmi SM, Quintiliano LA, Firoozabadi A. Polymeric dispersants delay sedimentation in colloidal asphaltene suspensions. Langmuir. 2010; 26(11): 8021-8029. doi: 10.1021/la9049204
- Hu YF, Guo TM. Effect of the structures of ionic liquids and alkylbenzenederived amphiphiles on the inhibition of asphaltene precipitation from CO2-injected reservoir oils. Langmuir. 2005; 21(18): 8168-8174. doi: 10.1021/la050212f
- Bai L, Nie Y, Li Y, Dong H, Zhang X. Protic ionic liquids extract asphaltenes from direct coal liquefaction residue at room temperature. Fuel Processing Technology. 2013; 108: 94-100. doi: 10.1016/j.fuproc.2012.04.008
- Junaki E, Ghanaatian S, Zargar G. A new approach to simultaneously enhancing heavy oil recovery and hindering asphaltene precipitation. Iranian Journal of Oil & Gas Science and Technology. 2012; 1(1): 37-42. doi: 10.22050/ijogst.2012.2773
- Ogunlaja AS, Hosten E, Tshentu ZR. Dispersion of Asphaltenes in Petroleum with Ionic Liquids: Evaluation of Molecular Interactions in the Binary Mixture. Industrial & Engineering Chemistry Research. 2014; 53(48): 18390-8401. doi: 10.1021/ie502672q



