Research Article
The impact of pH and Temperature on Gibbsite reactivity with Quartz
Department of Geosciences, Faculty of Geosciences and Petroleum Engineering, Universiti Teknologi Petronas (UTP), 31750, Tronoh, Perak, Malaysia
*Corresponding author: Eswaran Padmanabhan, Associate Professor, Department of Geosciences, Faculty of Geosciences and Petroleum Engineering, Universiti Teknologi Petronas, 31750, Tronoh, Perak, Malaysia, E-mail: eswaran_padmanabhan@utp.edu.my
Received: February 22, 2017 Accepted: April 7, 2017 Published: April 13, 2017
Citation: Ali AM, Padmanabhan E. The impact of pH and Temperature on Gibbsite reactivity with Quartz. Int J Petrochem Res. 2017; 1(1): 40-45. doi: 10.18689/ijpr-1000108
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In alumina industry, gibbsite (Al(OH)3) precipitation from sodium aluminate solution is
the foremost step in the production of alumina (Al2O3) from bauxite via the Bayer process. Hence, the precipitation of gibbsite has been extensively studied, with focus on kinetic
modelling of the growth and agglomeration of gibbsite under different batch precipitation
conditions. However, not much attention has been paid to gibbsite reactivity with quartz, given their ubiquitous nature. Stock solutions containing Alcl3 and quartz grains of varying
pH [5.5, 7.5 and 9] were prepared to establish optimum pH conditions for Al-oxides
precipitations. The synthesized gibbsite were characterized with FESEM, FTIR and Raman
spectroscopy. The competing processes of chemical leaching and dissolution-reprecipitation
between quartz and gibbsite showed that gibbsite is distinctly more crystallized with welldefined
polygonal structure at higher temperature (60°C) and low silica. The FESEM
micrographs showed that gibbsite can be synthesized in the pH range selected for this study, suggesting gibbsite is synthesizable as long as there are available OH- ions to hydrolyze Alcl3.
Keywords: Gibbsite; quartz dissolution; surface morphology; FTIR characterization
Introduction
Gibbsite (Al(OH)3) is generally formed from the chemical interactions between
weathered rocks and rainwater in hot and humid zones under high rainfall and high
leaching rates [1]. The ubiquitous nature of gibbsite in surface formations is generally
attributed to the action of high intensity weathering processes for a long duration [2]. This great abundance of gibbsite in soils and weathering products in tropical
environments is an indicative factor of advanced stages of weathering that are
characteristic of developed soils [3]. Gibbsite is even considered the definitive end
product of weathering [4]. Therefore, the study of hydrous gibbsite mineral at variable
pressure, pH and temperature conditions is crucial for understanding the dynamic
processes and circulation of gibbsite/aluminohydroxides bearing waters [5]. Moreover, gibbsite precipitation from sodium aluminate solution is the foremost step in the
production of alumina (Al2O3) from bauxite via the Bayer process [6], hence several
research has studied gibbsite precipitation with focus on kinetic modeling of the growth
and agglomeration of gibbsite under different batch precipitation conditions [7] [8] [9]. However, only a few studies have focused on developing the relationship between
gibbsite surface morphology and its growth mechanism [9]. Moreover, related studies
do not take into consideration that quartz and Al hydr (oxides) (gibbsite) are principal
components of silicaclastics, and the fact that field observations and experimental
studies have shown Al oxides form on quartz grains [10] [11].
The reactivity of quartz and gibbsite can act as a control in
the growth mechanism of gibbsite. This study is an attempt to
elucidate the surficial precipitation and growth mechanism of
gibbsite on quartz. Gibbsite reactivity with quartz will either inhibit
or catalyze the growth of Al hydr (oxides).This will improve the
understanding on the controls of gibbsite solubility. Therefore, the objective of this study is to determine the gibbsite morphology
at different pH and temperature conditions as well as to explore
the impact of quartz dissolution on gibbsite precipitation.
Materials and Methods
Gibbsite was synthesized on quartz substrates (qAl) by the slow addition of 0.5molL-1 KOH to 0.8molL-1 Alcl3. Stock solutions of the samples with varying pH [5.5, 7.5 and 9] were prepared. Afterwards, the stock solutions and their replicates were aged for 20days at room temperature (≈25°C) and 60°C. The pH was monitored daily for the 20 days (Fig. 1) to establish optimum pH conditions for Al-hydr (oxide) precipitation. The stock solutions were subsequently drained and the residual substrates dehydrated in an oven set at 40°C for 5 days. The amount of dissolved silica in the drained solution was measured using silica molybdate spectrophotometry method (HACH D2800). Fourier transform infra-red (FTIR) analysis was used to study quartz reactivity with gibbsite by identifying chemical bonds. Morphological variations of the synthesized gibbsite were analyzed using field emission scanning electron microscopy (FESEM). Pure quartz substrate was included in the experiment as a control sample [qAl0].
Results
pH and quartz dissolution measurements The daily pH measurements of the stock solutions show that pH declined sharply after 1 day of ageing, as presented in Fig.1. For measurements at pH9 (qAl9(60°C)), pH7.5(qAl7.5(60°C)), and pH5.5 (qAl5.5(60°C),) at 60°, the pH decreased sharply to pH4, pH3 and pH3.5, respectively. This decrease is more significant at pH 9. In addition, the decline in pH increases with increasing pH. In contrast, the pH declined less drastically in stock solutions stored at room temperature (qAl5.5, qAl7.5, qAl9) from 5.5, 7.5 and 9 to 6.5, 6.5 and 3.5, respectively. Nonetheless, the decline was similarly more significant at pH9. In stock solutions stored at 60°(qAl5.5(60°C), qAl7.5(60°C), qAl9(60°C)), the pH slightly increased after day 3, and then becomes steady for the remaining days, In contrast, no increase in pH is observed for solutions stored at room temperature.
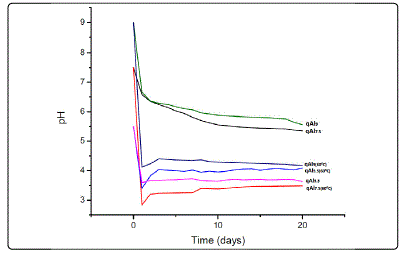
Figure.1: Plot showing variation in pH with time
The amount of dissolved silica was measured for each pH condition, as shown in Table. 1. As observed, the amount of dissolved silica expectedly increased from lower pH to higher pH at room temperature, with values of 9.5, 39.3, and 120 mg/L for qAl5.5', qAl7.5', and qAl9, respectively, given that quartz dissolves at low and high pH. At a higher temperature (60°), the dissolved silica in the system increased to as high as 180mg/L at pH 9 (Table. 1).

Figure.1: Dissolved silica at different synthesis conditions of gibbsite
FTIR characterization
FTIR spectra was obtained for the different synthesis
conditions, as shown in Fig. 2. The FT-IR peaks at approximately
775 and 1080cm-1 (Fig.2) denote Si-O quartz bonds, while the
peak at 1600cm-1 signifiesH-O-H bending of water. Al---O-H
stretching bond was absent. O-H stretching modes common
to most phyllosilicates lie in the spectral region of 3400 to
3750 cm-1. Metal-OH bending modes occur in the 600 to 950
cm-1 region. Si-O and Al-O stretching modes are found in the
700 to 1200 cm-1 range. Si-O and Al-O bending modes
dominate the 150 to 600 cm-1 region. Four bands at 914, 972, 1021 and 1060 cm-1 correspond to OH-bending vibrations. Most probably, the lower frequency band at 914 cm-1 is
attributed to the Al-O-H group with the least hydrogen
bonding influence. The bands in the 500 to 650 cm-1 region
are overlaps of out-of-plane OH bending vibrations and Al-O
vibrations.
In addition, Fig. 2 shows FTIR absorption bands at
approximately 3400cm−1 in the IR spectra of qAl5.5, qAl7.5, qAl9, qAl5.5(60°C), qAl7.5(60°C), and qAl9(60°C (Fig. 2) associated with
stretching vibrations of Al–OH groups and inter-layered
water, which indicate that OH groups are tetrahedrally
coordinated with Al+3 ions in gibbsite [2] [12]. However, as
observed in the spectra (Fig. 2), the cusp of the absorption
bands broadens (decreasing% transmittance) with reducing
pH. Moreover, the intensity of the absorption bands varies, where gibbsite samples synthesized at 60°exhibitrelatively
lower % transmittance compared to their room temperature
counterpart.
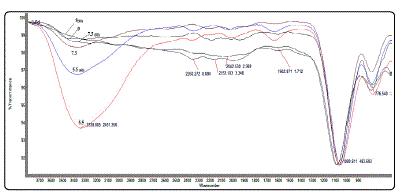
Figure.2: Overlapping FTIR spectra of the different synthesized gibbsite
Raman analysis of gibbsite coated quartz
Raman measurements in the range of 50-1250 cm-1 reveal
distinct bands with frequencies and relative intensities. Regardless of the similarity in the character of the pure quartz and gibbsite coated quartz substrates spectra, there exists a
variation in the positions and intensity of the similarly shaped
peaks for the two phases. The distinctive strong quartz peak
of pure quartz substrates (q0) at 465cm-1 deviates slightly to
463cm-1 peak in the spectra of gibbsite coated quartz
substrates (qAl) as shown in Fig. 3. The shift indicates tensile
stress denoted by the slight broadening of the peak, invariably
suggesting reduced crystallinity of the quartz. Other identified
Raman shifts in the pure quartz include peaks at 352, 400 and
1137cm-1. The variation in intensity points to the effect
occlusion. The 465cm-1 peak of pure quartz grains show an
intensity of 420 cm-1, while that of quartz grains occluded with
gibbsite are characterized by a relatively lower intensity. The
decadence of the erstwhile prominent quartz peak indicates
reduced crystallinity. In addition, quartz is observed to have a
broad low-frequency Raman band at 199cm-1, which is
apparent in gibbsite coated quartz substrates. The broadness
of this band has been attributed to the anharmonic coupling
of an A1 phonon with a two-phonon (acoustic) mode [13].
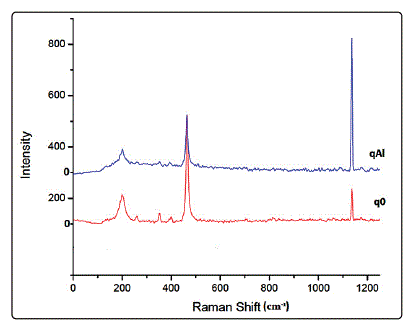
Figure.3: Raman spectra of pure quartz (q0) and gibbsite coated quartz (qAl)
Morphological characterization
The morphology of the gibbsite coated quartz was
characterized using FESEM. Observation of samples
synthesized at room temperature shows that the synthesized
gibbsite is constituted by well-spaced clustered micrometer
sized particles, (Fig. 4A) with no tendency to form aggregates. This property of not forming aggregates is directly related to
the free-flowing characteristics of gibbsite. A second observed
feature is the peculiar morphology of each particle, shown in
higher magnification in Fig. 4B. Deep desiccation cracks occur
after the growth of gibbsite, apparently attributed to the
dehydration of the sample (Fig. 4C). The free-flowing
precipitated gibbsite is depicted as lettuce shaped terminations
[14] (Fig. 4D). Each particle is an agglomerate of platy crystals, with cubic terminations. It should be noted that the
precipitated material coats the entire quartz substrate making
the quartz surface indiscernible.
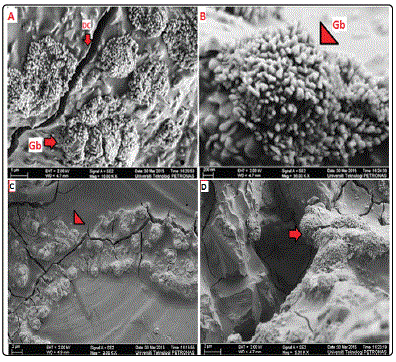
Figure.4: A) well separated clusters of synthesized gibbsite attributable to its free-flowing characteristic (G) B) distinct lettuce shape: infilling of gouges and notches on the quartz surface with agglomerated gibbsite clusters C) desiccation cracks D) gibbsite terminations on quartz edges
The samples synthesized at 60°C were also characterized. The morphology shows protuberances a top the quartz grains. The initially dispersed quartz grains become cemented by the crystallized gibbsite (Fig. 5A). A closer view (Fig. 5B) shows the quartz grains agglomerated by gibbsite. Fig. 5C shows the appearance of deeper and more prominent desiccation cracks on the gibbsite cement caused by the intensive dehydration process. At 60°C, the initial distinct lettuce shaped gibbsite crystals are transformed into well-defined hexagonal gibbsite crystal growths and prismatic laths as long as 3µm, as shown in Fig. 5D. The gibbsite prismatic crystals are arranged in overlapping layers.
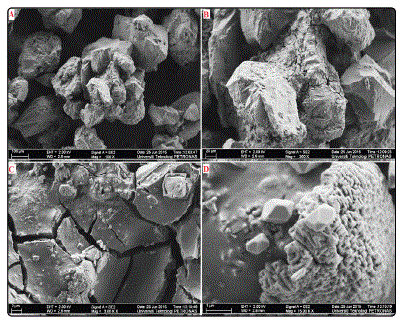
Figure.5: FESEM micrographs showing A) agglomerations of quartz grains B)cementation of quartz by gibbsite C) deeper and more prominent desiccation cracks D) highly crystallized polygonal gibbsite gibbsite crystals
The sample with the highest dissolved silica (qAl9(60°C)) was further characterized, as shown in Fig. 6. The dissolved silica is leached over the synthesized gibbsite. This makes the gibbsite crystals inconspicuous.
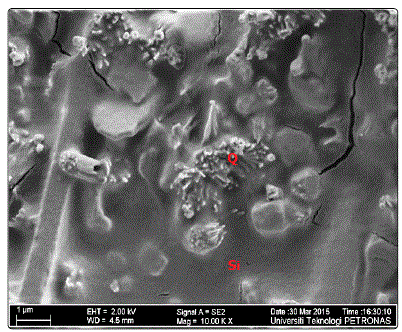
Figure.6: FESEM micrograph of qAl9(60°C
Discussion
Effect of pH and temperature
AlCl3 was hydrolyzed on quartz substrates to form
insoluble colloidal and stable suspensions of aluminum oxides
confirmed by the FTIR. The pH of the mother liquor controls
the structure of the aluminum hydroxide precipitate. The
variation in pH with time can be explained by the following
mechanism. The trivalent compound [Aluminium chloride]
basically hydrolyzes in water solution and ionizes to aluminium
and chloride ions; whilst the water ionizes to hydrogen and
hydroxide ions as illustrated by Eqs. (1) and (2); making the
solution strongly acidic. The compounds are denoted with the
subscript 'M'. Afterwards, the aluminium ions partially
combines with the hydroxide ions released from the
dissociation of water to form aluminium hydroxide (Eq.3), a
compound which is only slightly soluble and precipitates from
solution as a whitish solid. This process is catalyzed by heating
up the solution and adding a base.
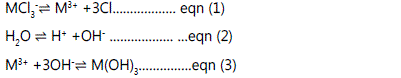
As a base is added to the AlCl3 solutions, the OH- ions are
deplete and neutralize the available H+ ions to form water, causing the pH value to rise until the pH of the base being
added is attained. The further addition of a base has little
effect, with the pH remaining relatively constant based on the
excess amount of OH- in the system. Within this range, OHbecomes
constantly absorbed of by Al cations overtime to
form oxy-hydroxide species. Thus, the OH- ions are neutralized
by the trivalent and H+ ions, leading to the decline in pH. However, the pH of the null sample (pure quartz) remain
constant at 12.5 possibly due to the absence of neutralizing
agents.
The FESEM micrographs showed that gibbsite can be
synthesized in the pH range selected for this study. This
suggests gibbsite can be synthesized as long as there are
available OH- ions to hydrolyze Alcl3. At room temperature, the synthesized gibbsites are basically lettuce shaped
terminations. However, for stock solutions stored at a higher
temperature (60°C), the gibbsite becomes more crystallized
with well-defined polygonal structure. Thus, it can be inferred
that the synthesis of gibbsite at 60°Cenhances its crystallinity
without deforming crystal structure. This is consistent with the
FTIR and Raman analyses, where the hydroxyl bands of
samples stored at room temperature (25°C) exhibited lower
%transmittance,due to water molecules locked in their crystal
structure, compared to those synthesized at 60°C.
Quartz reactivity with Gibbsite
The bulk of quartz is composed of Si-O-Si [siloxane bonds]
linkages, although the surface terminations consist of
hydrophilic hydroxyl groups [terminal OH] [15], since the lowcoordinated
metal cations at the quartz surface are able to
undergo hydrolysis in the presence of the water molecules to
produce hydroxide layers (surface hydroxyls: -Si-OH) [16], as
shown in Eq. (3). These hydroxide sites are very reactive, in
that a proton from the adjacent solutions may be accepted or
removed from the mineral surface, i.e. the silica surfaces can
interact with water in two competing processes: physiochemical
adsorption by hydrogen bonding (protonation) and chemical
dissolution [17] expressed in Eqs. (4) and (5), respectively.
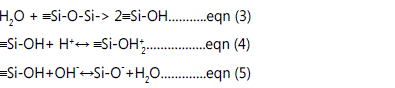
The adsorption process causes the oxygen of the water molecule to form a H-bond with the hydrogen of the OH surface group [OH coordinated to single silicon: Si-OH] as shown below in Fig. 7, based on proposed ab initio calculations [18]. Conversely, the water molecules are able to interact with the surface to cleave the Si-O-Si linkages [siloxane], resulting in hydrolyzed products [SiO-], where a proton can be lost from the surface OH (deprotonation), usually in a basic solution [19][20][21]. Therefore, quartz dissolution is dependent on the hydrolysis of surface complexes. Theoretically, the presence of trivalent ions like Al3+ on quartz dissolution presents a different scenario. This study hypothesizes that the formation of Al complexes (Al (OH)3) on the quartz surface, where the trivalent cations adsorb to SiO- ligands to form new cationic ligands capable of stabilizing the quartz surface by combining with the surface Si- OH- and dangling OH-to form Al silicates and Al hydroxides, as depicted in Fig. 7.Although quartz is a nonreactive surface, it can serve as a media for gibbsite synthesis. The competing processes of chemical leaching and interfacial dissolution-reprecipitation between different materials are shown, where gibbsite is less distinct in solutions containing high dissolved silica (q Al5.5(60°C)). Al hydr (oxides) coat the quartz surface and creates a surficial secondary layer that can allow the adsorption and agglomeration of other minerals.
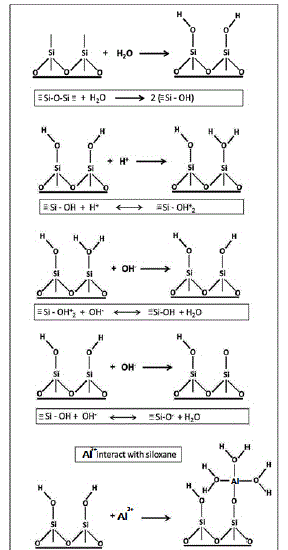
Figure.7: Model of quartz dissolution and Al3+ interaction with quartz
Synthesis of gibbsite
As shown in the FESEM images, gibbsite is a more efficient
coating and cementing material at high temperature, which
has been attributed to the increased planar form of Al
hydroxide particles [22]. Thus, Al oxides will be efficient at
reducing swelling and dispersion of clay as well as initiate a
greater flocculent effect in a high temperature system, thereby
improving soil hydraulic conductivity and tensile strength as
suggested in earlier studies [23] [24]. The distribution of
gibbsite is dependent on rapid precipitation or hydrolysis of
Alcl3 or other Al bearing compounds, pH, as well as
temperature. The formation of gibbsite can retard quartz
dissolution or resilication to a more stable kaolinite under a
given H4SiO4 activity, while intense leaching at high
temperatures in the presence of dissolved silica (H4SiO4) activity may enhance the formation of more stable clay
minerals (kaolinite and smectite).
For industrial gibbsite precipitation, the product specified
particle size distribution and morphology can be achieved by
controlling the pH and temperature of gibbsite agglomeration
and growth processes. At the microscopic level, both gibbsite
agglomeration and growth occur on the crystal surface. This
paper provides valuable insights into the growth mechanisms
of gibbsite on quartz, which have direct influence on the
particle growth pathways and reaction kinetics. Taking
advantage of this new insight may bring about improvements
in the crystallization technology of gibbsite, which is presently
a sluggish process that requires trains of massive stirred tanks
[9]. Thus, optimum conditions for synthesizing gibbsites in the
presence of quartz include near neutral pH solutions at
approximately 5.5 and temperature of 60°C, i.e.qAl5.5(60°C).
Conclusion
The competing processes of chemical leaching and dissolution-reprecipitation between aluminium hydr (oxides) and quartz are shown, where gibbsite is distinctly more crystallized at higher temperature and low silica system. Thus, dissolved silica inhibits the crystallization of gibbsite minerals. Nonetheless, the precipitated Al hydr (oxides) coats the quartz surface and creates a surficial secondary layer that can possibly allow adsorption and agglomeration of other compounds. Gibbsite was precipitated within the pH range of 5 to 9, suggesting gibbsite can be synthesized as long as there are available OH- ions to hydrolyze Alcl3 and other Al bearing compounds.
Acknowledgement
This work was supported by the Petroleum Research Fund awarded to E. Padmanabhan.
References
- Mutakyahwa MKD, Ikingura JR, Mruma AH. Geology and geochemistry of bauxite deposits in Lushoto District, Usambara Mountains, Tanzania. Journal of African Earth Sciences. 2003; 36(4): 357-69. doi: 10.1016/ S0899-5362(03)00042-3
- Tchakoute HK, Rüscher CH, Djobo JNY, Kenne BBD, Njopwouo D. Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Applied Clay Science. 2015; 107: 188-194. doi: 10.1016/j.clay.2015.01.023
- Vazquez FM. Formation of gibbsite in soils and saprolites of temperaturehumid zones. Clay Minerals. 1981; 16(1): 43-52.
- Funakawa S, Watanabe T, Kosaki T. Regional trends in the chemical and mineralogical properties of upland soils in humid Asia: With special reference to the WRB classification scheme. Soil Science and Plant Nutrition. 2008; 54(5): 751-60. doi: 10.1111/j.1747-0765.2008.00294.x
- Huang PM, Wang MK, Kampf N, Schulze DG. Aluminum hydroxides. In 'Soil Mineralogy with Environmental Applications'. Soil Science Society of America: Madison. 2002.
- Fu W, Vaughan J, Gillespie A. Effects of inorganic anions on the morphology of sodium oxalate crystallized from highly alkaline solutions. Crystal Growth & Design. 2014; 14 (4): 1972-80. doi: 10.1021/cg5000952
- Fu W, Vaughan J, Gillespie A. Aspects of the mechanism of nucleation and intergrowth of gibbsite crystals on sodium oxalate surfaces in concentrated alkaline solutions. Crystal Growth & Design. 2015a; 15(1): 374-83. doi: 10.1021/cg501465v
- Fu W, Vaughan J, Gillespie A. In situ AFM investigation of heterogeneous nucleation and growth of sodium oxalate on industrial gibbsite surfaces in concentrated alkaline solution. Chemical Engineering Science. 2015b; 126: 399-405. doi: 10.1016/j.ces.2014.12.057
- Fu W, Vaughan J, Gillespie A. In situ AFM investigation of gibbsite growth in high ionic strength, highly alkaline, aqueous media. Hydrometallurgy. 2016; 161: 71-76. doi: 10.1016/j.hydromet.2016.01.030
- Coston JA, Fuller CC, and Davis JA. Pb2+ and Zn2+ adsorption by a natural aluminum- and iron-bearing surface coating on an aquifer sand. Geochimica et Cosmochimica Acta. 1995; 59: 3535-47. doi: 10.1016/0016- 7037(95)00231-N
- Davis JA, Coston JA, Kent DB, Fuller CC. Application of the surface complexation concept to complex mineral assemblages. Environmental Science & Technology. 1998; 32(19): 2820-28. doi: 10.1021/es980312q
- Schroeder PA. Infrared Spectroscopy in clay science: In CMS Workshop Lectures , Teaching Clay Science, edited by Rule, A and Guggenheim, S, (The Clay Mineral Society,Aurora, Colorado)2002; 11: 181-206.
- Jayaraman A, Kourouklis GA, Espinosa G P Cooper AS, VanUitert LG. A high-pressure Raman study ofyttrium vanadate (YVO.) and the pressureinduced transition from the zircon-type to the scheelite-type structure. Journal of Physics and Chemistry of Solids. 1987; 48(8): 755-59. doi: 10.1016/0022-3697(87)90072-2
- Pedro KK, Helena SS, Antonio C, Vieira C, Pérsio de SS. Structure, surface area and morphology of aluminas from thermal decomposition of Al (OH) (CH3COO)2 crystals. Anais da Academia Brasileira de Ciências. 2000; 72(4): 471-495. doi: 10.1590/S0001-37652000000400003
- Nangia S, Garrison BJ. Reaction rates and dissolution mechanisms of quartz as a function of pH. J. Phys. Chem.2008; 112(10): 2027-33. doi: 10.1021/jp076243w
- Degen A, Kosec M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. Journal of the European Ceramic Society. 2000; 20(6): 667-73. doi: 10.1016/S0955-2219(99)00203-4
- Nangia S,Washton NM, Mueller KT, Kubicki, JD, Garrison B. Study of a family of 40 hydroxylated β-cristobalite surfaces using empirical potential energy functions. J. Phys. Chem B. C. 2007; 111: 5169-177. doi: 10.1021/jp0678608
- Lasaga AC, Gibbs GV. Ab ibitio quantum mechanical calculations of surface reactions – A new era?. In Aquatic chemical studies, edited by W. Stumm (ed.), John Wiley and sons, New York. 1990a; 259-89.
- Xiao Y, Lasaga AC. Ab initio quantum mechanical studies of the kinetics and mechanisms of quartz dissolution: OH catalysis. Geochimica et Cosmochimica Acta. 1996; 60(13): 2283-95. doi: 10.1016/0016- 7037(96)00101-9
- Pelmenschikov A, Strandh H, Pettersson LGM, Leszczynski J. Lattice resistance to hydrolysis of Si–O–Si bonds of silicate minerals: ab initio calculations of a single water attack onto the (001) and (111) β-cristobalite surfaces. J. Phys. Chem B. 2000; 104(24): 5779-783. doi: 10.1021/jp994097r
- Criscenti LJ, Kubicki JD, Brantley SL. Silicate glass and mineral dissolution: calculated reaction paths and activation energies for hydrolysis of a Q3 Si by H3O+ using ab initio methods. J. Phys. Chem A. 2006; 110: 198-206. doi: 10.1021/jp044360a
- El-Swaify SA, EmersonWW. Changes in the physical properties of soil clays due to precipitated aluminum and iron hydroxides: I. Swelling and aggregate stability after drying. Soil Science Society of America, Proceedings. 1975; 39: 1056-1063. doi: 10.2136/ sssaj1975.03615995003900060016x
- El-Rayah, HME, Rowell DL. The influence of iron and aluminium hydroxides on the swelling of Namontmorillonite and the permeability of a Na-soil. European Journal of Soil Science. 1973; 24(1): 137-144. doi: 10.1111/j.1365-2389.1973.tb00749.x
- Goldberg S, Glaubig RA. Effect of saturating cation, pH and aluminum and iron oxides on the flocculation of kaolinite and montmorillonite. Clays & Clay Minerals. 1987; 35: 220-27.



