Research Article
Adsorption of Chemically Prepared Cocoa Nibs Based Activated Carbon Onto Methylene Blue: Equilibrium and Kinetic Studies
1Faculty of Science and Technology, Universiti Sains Islam Malaysia, 71800 Nilai, Negeri Sembilan, Malaysia
2Jabatan Perubatan Forensik, Hospital Kuala Lumpur, 50586 Kuala Lumpur, Malaysia
3Cocoa Innovative and Technology Centre, Malaysian Cocoa Board, Lot 12621, 71800 Nilai, Negeri Sembilan, Malaysia
4Academy of Language Studies, Universiti Teknologi MARA Pahang, Bandar Tun Abdul Razak, Jengka, Pahang, Malaysia
5School of Chemical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 NibongTebal, Penang, Malaysia
6Solid Waste Management Cluster, Science & Engineering Research Centre, Engineering Campus, Universiti Sains Malaysia, Nibong Tebal, Penang, Malaysia
*Corresponding author: Mohd Azmier Ahmad, Associate Professor, School of Chemical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia, Tel: +604 5996459, Fax: +604 5996908, E-mail: chazmier@usm.my
Received: March 23, 2017 Accepted: April 4, 2017 Published: April 10, 2017
Citation: Nikman KA, Ahmad F, Hassan MS, Nikman K, Ahmad MA. Adsorption of Chemically Prepared Cocoa Nibs Based Activated Carbon Onto Methylene Blue: Equilibrium and Kinetic Studies. Int J Petrochem Res. 2017; 1(1): 15-18. doi: 10.18689/ijpr-1000104
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study was aimed to prepare cocoa nibs based activated carbon (CNAC) via
chemical activation for methylene blue (MB) dye adsorption from aqueous solution. The
activation process was performed at 500°C under inert condition using K2CO3 as
activation agent. The effect of contact time and initial concentration of adsorbate on the
adsorption process were examined. Langmuir isotherm model fitted well the adsorption
equilibrium data with monolayer adsorption capacity of 64.98 mg/g at 30°C. The
adsorption kinetic was found to follow the pseudo-second-order kinetic model.
Keywords: Activated carbon, Chemical activation, Cocoa nibs, Methylene blue.
Introduction
Water pollution has become a highlighted issue as some of the industrial effluent is
directly discharged into the river and water bodies without proper treatment [1]. Dyes
effluent treatments from textile industries are divided into biological, physical and
chemical treatment processes. All these processes have different color removal
capabilities, capital costs and operating rates [2]. Physical treatment via adsorption
process using Agrowaste based activated carbon as adsorbent is among the most
efficient and cheap method for dyes removal. Several studies on agrowastes have been
employed to remove the contaminants such as methylene blue (MB) dye from the water
bodies [3] [4]. Agrowaste is appreciated as economical, sustainable and ecologically
friendly materials. In this study, an attempt was made to utilize cocoa nibs waste into
activated carbon using chemical activation process. The performance of CNAC was
studied in removing MB dye from aqueous solution.
Experimental
Chemicals
All reagents and chemicals used in the research were analytical grade chemicals. For
impregnation process, potassium carbonate (K2CO3) was used. Commercial methylene
blue (C16H18ClN3S·3H2O) (MB) textile dye was obtained from Sigma-Aldrich (M)
Sdn Bhd
Preparation of Stock and Test Solutions
Stock solution of MB with concentration of 500 mg/L was
prepared by dissolving approximately 0.50 g of MB in 1000 mL
distilled water. Test solution of MB ranging from 10 mg/L to
100 mg/L were prepared by subsequent dilution from stock
solution.
Production of Pellet
The cocoa nibs waste was ground and sifted to a uniform
size of 0.5 mm or smaller. The samples were shaped into
pellets by compressing the mixture with a hydraulic press, in
which the cocoa nibs were compacted into pellets with a
specific density of 1.8 g/cm.
Preparation of Activated Carbon
The carbonization was performed by placing approximately
60 g of cocoa nibs pellet in a vertical furnace, which contained
in a tubular stainless steel reactor. This step was carried outat
700°C for 1 hour under purified nitrogen (99.99%) with flowrate
of 120 mL/min. Then, the furnace was allowed to cold to ambient
temperature. Char yield was determined using following
equation:
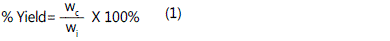
where is the mass of char after carbonization process and wi is the initial mass of cocoa nibs pellet. An amount of dried char was added with K2CO3 at various impregnation ratio (IR) in a 250 ml beaker. Both were mixed with deionized water to dissolve the salt. The IR was calculated as follows:
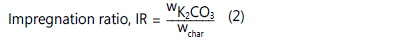
where wK2CO3
is the dry weight of potassium carbonate
pellets and wchar is the dry weight of cocoa nibs char. The mixture
was then dehydrated in an oven at 105°C for 24 hours. The
activation step was done using similar reactor as in carbonization
step but at final temperature of 500°C under nitrogen flowrate of
120 mL/min for 2 hours. The sample was then cooled to room
temperature and washed with hot deionized water until the pH
of the washed solution reached 6.5-7.
Removal of MB by Batch Adsorption Study
The batch equilibrium experiments of the adsorption
capacity studies were conducted at 30 °C in a 250 mL conical
flask in a water bath shaker. The stock solution (1000 mg/L) of
MB was prepared by dissolving approximately weighed 1.0 gram
of the MB in 1000 mL of distilled water in a 1500 mL volumetric
flask. The stock solutions were stored in dark place to prevent
direct sunlight. The sample solutions were withdrawn at equilibrium
to determine the residual concentration. The concentrations of
the filtrates were measured using UV-Visible Spectrophotometer (Model Agilent Cary 60, USA).
Effect of Initial Concentration
In order to study the effects of initial adsorbate concentration, 100 mL of adsorbate solutions with known initial concentration (10, 25, 50, 80, 100 mg/L of MB) were prepared in a series of
250 mL Erlenmeyer flasks. The amount of adsorbent that was
added into each flask was fixed at 0.1 g. The flasks were placed in an isothermal water bath shaker at temperature and
rotation speed of 30°C and 120 rpm, respectively until
equilibrium point was reached.
Effect of Contact Time
In order to study the effects of contact time on the
adsorption uptake, 100 mL of adsorbate solutions with known
initial concentration (10-100 mg/L) were prepared in a series
of 250 mL Erlenmeyer flasks. The amount of CNAC that was
added into each flask containing MB solution was fixed at 0.1
g. The flasks were then placed in an isothermal water bath
shaker of 30 °C with rotation speed of 120 rpm for 360
minutes. The percent removal of adsorbate was calculated as
follows:
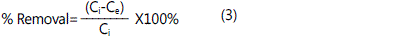
Where Ce is the concentration of adsorbate at equilibrium and Ci is the initial concentration of adsorbate. The MB uptake at equilibrium is calculated as follows:

where m is the mass of the adsorbent and V is the volume
of the adsorbate.
Adsorption Isotherm
Adsorption isotherm study was carried out by fitting the
equilibrium data to three isotherm model which are the
Langmuir and Freundlich isotherm models, respectively
represented by Equations (5),(6) and (7). The applicability and
suitability of the isotherm equation to the equilibrium data
was compared by judging the values of the correlation
coefficients, R2. The Langmuir model is atypical model used to
measure the amount of adsorbate on an adsorbent at
equilibrium. The relation is expressed by following equation
[5]:
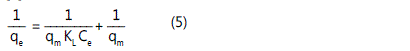
where, qe is the amount adsorbed (mg/g), Ce is the
equilibrium concentration of the metal ion (mg/L), qe is the
maximum amount of adsorbed metal ion per unit mass of
sorbent corresponding to complete coverage of the adsorptive
sites(mg/g), KL is the Langmuir constant related to the energy
of adsorption(L/mg)
The Freundlich adsorption isotherm or Freundlich
equations are a relation between the concentrations of a
solute on the surface of an adsorbent, to the concentration of
the solute in the liquid with which it is in contact. The
relationship is stated as follows [6]:
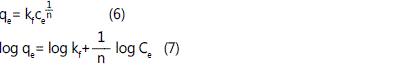
In this equation, qe (mg/g) is amount of adsorbed material
in adsorbent surface k in arrangement are adsorption capacity
and adsorption intensification.
Kinetic Model
The kinetics of adsorption describes the rate of adsorbate
uptake on activated carbon prepared and it controls the
equilibrium time. The kinetics of adsorbate uptake is required for
selecting optimum operating conditions for the full-scale batch
process. Therefore, models for liquid-phase adsorption such as
pseudo-first-order and pseudo-second-order were used to
analyze the adsorption kinetic data. The pseudo-first-order kinetic
model equation of Largergren [7] is generally expressed as:

Where qe is the amount of adsorbate adsorbed at equilibrium (mg/g), qt is the amount of solute adsorb per unit weight of adsorbent at time (mg/g), k1 is the rate constant of pseudo-first order sorption (1/h). The pseudo-second-order equation is expressed as [8]:
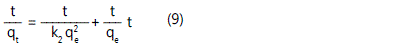
Results and Discussion
Effect of contact time and MB initial concentration on
adsorption equilibrium
Figure 2 and Table 1 show the effect of various initial
concentrations on adsorption of MB by CNAC. An equilibrium
time of 100 min was needed for MB dye solution with initial
concentrations of 10-25 mg/l to reach equilibrium. However, for initial concentrations of 50-100 mg/l, longer equilibrium
times of 22-24 hours were required for the system to reach
equilibrium. Initially, adsorbate molecules have to first
encounter the boundary layer effect. Then it has to diffuse
from boundary layer film onto adsorbent surface and finally, it
has to diffuse into the porous structure of the adsorbent [10]. The ratio of the initial number of dye molecules to the
available surface area was low at lower initial concentration
compared to higher initial concentration. Therefore, MB
solution with higher initial concentration would take relatively
longer contact time to attain equilibrium due to the higher
amount of MB molecules.
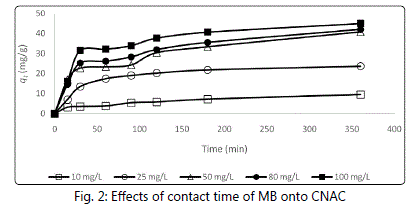
Figure 2: Effects of contact time of MB onto CNAC
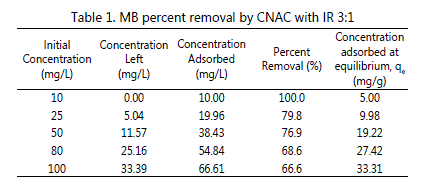
Table 1: MB percent removal by CNAC with IR 3:1
Effect of IR on adsorption equilibrium
Fig. 1 shows the influence of IR on CNAC in removing MB. Sample impregnated with 2:1 ratio has the lowest capability
to adsorb the adsorbate where the value is 27.8 mg/g.
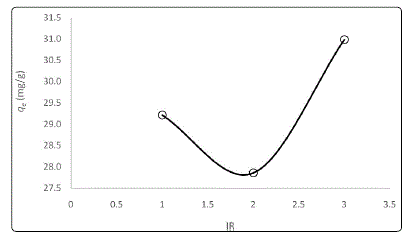
Figure 1: Effect of IR on MB uptake
The adsorption capacity of CNAC was decreased with
increased in IR from 1:1 to 2:1 before increased at IR of 3:1. CNAC with 3:1 ratio managed to adsorb 30.9 mg/g of MB at
equilibrium. Higher IR was favorable for enhancing the MB
adsorption as more pores are developed on the sample
surface [9].
Adsorption Isotherm
The most appropriate correlation for the equilibrium
curve needs to be established in order to understand the
adsorption system. Therefore, the equilibrium adsorption
data were analyzed using the Langmuir and Freundlich
isotherms as shown in Figure 3 whereas Table 2 summarizes
all the constants from both isoterm models.
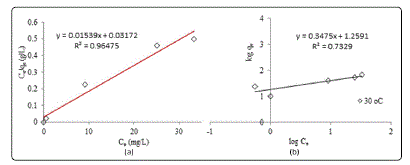
Figure 3: Linear plots of (a) Langmuir and (b) Freundlich adsorption models
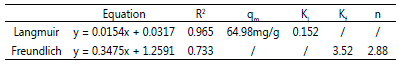
Table 2: Parameters of Langmuir and Freundlich adsorption isotherm for MB.
Langmuir model gave the highest R2 values which were
greater than 0.96. Conformation of the experimental data into
the Langmuir isotherm equation proved that the surface of
CNAC for MB adsorption was made up of homogeneous
adsorption patches with monolayer coverage of MB onto
CNAC [9]. The monolayer saturation capacity of CNAC was
found to 64.98 mg/g at 30 °C.
Adsorption Kinetics
All the experimental and calculated qe values obtained
from the pseudo-first-order and pseudo-second-order kinetic
model for adsorption of MB at 30 °C are tabulated in Table 3. Comparing the R2 values, pseudo-second-order model
showed a significant agreement with adsorption mechanisms, which indicated the chemisorption with the heterogeneous
active sites occurred on the surface of CNAC [11].
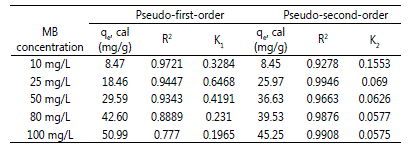
Table 3: Parameter values of the kinetic studies of the adsorption of MB onto CNAC.
Conclusion
CNAC was found to be suitable for the removal of MB from aqueous solution. Adsorption of MB was found to increase with increase in contact time and initial dye concentration. The Langmuir isotherm model was well described by the equilibrium data with maximum adsorption capacity of 64.98 mg/g. The pseudo-second-order kinetic model fits well with the kinetic data.
Acknowledgment
The authors are grateful for the funding from Solid Waste Management Cluster, USM (1001/CKT/870023) and research grant from Ministry of Science, Technology and Innovation (MOSTI), Malaysia.
References
- Afifah N, Saleh R. Removal of methylene blue dye contaminant by combination of ultrasonic and visible light irradiation using perovskite LaMnO3 nanocatalyst. Materials Science Forum. 2016; 864: 99-105.
- Amin N. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. Haz Mater J. 2009; 165(1-3): 52-62. doi: 10.1016/j.jhazmat.2008.09.067
- Ghaedi M, GolestaniNasab A, Khodadoust S, Rajabi M, Azizian S. Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. Ind Eng Chem J. 2014; 20(4): 2317-2324. doi: 10.1016/j.jiec.2013.10.007
- Kumar A, Jena HM. Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. Cle Prod J. 2016; 137: 1246-1259. doi: 10.1016/j.jclepro.2016.07.177
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemistry Society. 1918; 40(9): 1361- 1403. doi: 10.1021/ja02242a004
- Freundlich HMF. Over the adsorption in solution. Journal of Physical Chemistry. 1906; 57: 385-470.
- Langergren S, Svenska BK. Zurtheorie der sogenannten adsorption geloesterstoffe, Veternskapsakad. Handlingar. 1898; 24: 1-39.
- Ho YS, McKay G. Sorption of dye from aqueous solution by peat. Chem Engg J. 1998; 70(2): 115-124. doi: 10.1016/S0923-0467(98)00076-1
- Ahmad MA, Rahman NK. Equilibrium, kinetics and thermodynamic of Remazol Brilliant Orange 3R dye adsorption on coffee husk-based activated carbon. Chem Engg J. 2011; 170(1): 154-161. doi: 10.1016/j. cej.2011.03.045
- Tan IAW, Ahmad AL, Hameed BH. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. Haz Mater J. 2009; 164(2-3): 473-482. doi: 10.1016/j.jhazmat.2008.08.025
- Ahmad F, Daud WMAW, Ahmad MA, Radzi R. Using cocoa (Theobroma cacao) shell-based activated carbon to remove 4-nitrophenol from aqueous solution: Kinetics and equilibrium studies. Chem Engg J. 2011; 178: 461-467. doi: 10.1016/j.cej.2011.10.044



