Research Article
Effect of pyrolysis temperature on gas permeation characteristics of poly(p-phenylene oxide) derived hollow fiber carbon membranes
1School of Chemical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia
2Membrane Science and Technology Cluster, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia
3Advanced Membrane Technology Research Centre, Universiti Teknologi Malaysia, 81310 Skudai, Johor, Malaysia
4Solid Waste Management Cluster, Science & Engineering Research Centre, Engineering Campus, Universiti Sains Malaysia, Nibong
Tebal, Penang, Malaysia
*Corresponding author: Mohd Azmier Ahmad, Associate Professor, School of Chemical Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 NibongTebal, Penang, Malaysia, E-mail: chazmier@usm.my
Received: February 24, 2017 Accepted: March 31, 2017 Published: April 7, 2017
Citation: Jaya MAT, Harun WMHW, Yusop MFM, Ismail AF, Ahmad MA. Effect of pyrolysis temperature on gas permeation characteristics of poly(p-phenylene oxide) derived hollow fiber carbon membranes. Int J Petrochem Res. 2017; 1(1): 12-14. doi: 10.18689/ijpr-1000103
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Hollow fiber carbon membranes derived from poly(p-phenylene oxide) were subjected to different pyrolysis temperatures and their corresponding gas permeation characteristics were studied. Carbon membrane produced using pyrolysis temperature of 500°C was low in H2, O2, N2 and CH4 permeabilities, as well as H2/N2 and O2/N2 ideal selectivities. The highest permeabilities and ideal selectivities were obtained at 600°C. Different trend was shown by the CO2 permeability which increased with pyrolysis temperature before decreased at 700°C. The CO2/CH4 ideal selectivity consistently increased with increasing pyrolysis temperatures.
Keywords: Poly(p-phenylene oxide), Hollow fiber, Carbon membrane, Pyrolysis temperature, Gas separation.
Introduction
Carbon membranes possess the capability of discriminating gas molecules with similar kinetic diameters such as O2 and N2 [1]. This feature has attracted many researchers to investigate and further develop the carbon membranes into commercially acceptance. Currently, the carbon membrane development focuses on finding new precursors and modification of the existing precursors. The precursors were mostly polyimides which is economically unattractive, thus non-polyimide polymers such as phenolic resin [2], cellulose acetate [3] and poly(furfuryl alcohol) [4] were extensively introduced. The current study reports interesting findings on the ideal gas separation characteristics of hollow fiber carbon membrane produced from poly(p-phenylene oxide) (PPO) using different pyrolysis temperature.
Methods
The hollow fiber PPO and carbon membranes were synthesized according to the previous work [5]. Heat treatments which were thermostabilization and pyrolysis, were applied to convert the PPO membrane into carbon membrane. The thermostabilization temperature was 240°C and held for 1 hour before proceeding to pyrolysis treatment at varied temperatures. A constant pressure/variable-volume system and a soap-bubble flow meter were used to measure the membrane flow rate. The membrane module was open/close-ended in which gas was fed from the membrane bore side. The order of the feed was H2, N2, CO2, CH4 and CO2 to prevent strong adsorbing gases from influencing the measurement of the proceeding gases [6]. The morphology of the carbon membrane was captured using scanning electron microscope (SEM, Model Quanta FEG 450, FEI, USA).
Results and discussions
Fig. 1 (a–b) and (c–d) shows the cross-sectional morphology of PPO and carbon membranes, respectively. Both membranes exhibited homogeneous, symmetric, and nonporous structure. The PPO and carbon membranes were approximately 15.4 and 14.7 µm thick. The non-porous structure of the PPO membrane (PPOM) stemmed from the delayed phase inversion between the polymer solution and ethanol bore fluid and bath. The diameter and thickness of the membrane decreased after pyrolysis because of thermal shrinkage and decomposition. The change in surface morphology also suggested structure rearrangement during pyrolysis.
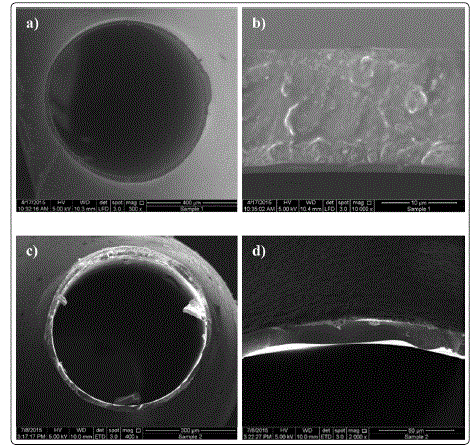
Figure 1: SEM images of cross-sectional views of PPO membrane (a, b) and carbon membrane (c, d)
Figure 2 shows the permeabilities of H2, CO2, O2, CH4 and N2 for carbon membranes pyrolyzed at 500°C (CM500), 600°C (CM600) and 700°C (CM700) with heating rate and thermal soak time of 1°C/min and 15 mins, respectively. PPOM permeabilities was included for comparison purpose. The permeabilities of H2, O2, N2 and CH4dropped significantly when the sample was pyrolyzed to 500°C. Similar trend was followed by their corresponding ideal selectivities of O2/N2 and H2/N2. In contrast, the CO2 permeability and CO2/CH2 ideal selectivity increased. As the pyrolysis temperature was increased to 600°C, all the gas permeabilities and ideal selectivities increased significantly. The ideal separation performances of the gases, except CO2/CH4 ideal selectivity, decreased again as the pyrolysis temperature was further increased to 700°C.
According to the values of permeabilities and ideal
selectivities, particularly O4/N4, of the hollow fiber carbon
membrane, the transport mechanism of the gases was most
probably dominated by the molecular sieving in which it
suggested that the membranes possessed micropore structure
with pore size less than 2 nm [7] [8]. When the thermostabilized
PPO membrane was subjected to pyrolysis treatment, slow
decomposition of the polymer structure took place. At the
same time, thermal shrinkage and structural rearrangement
also occurred. The amorphous structure originated from the
thermostabilized PPO membrane gradually collapsed during
the pyrolysis and chaotically rearranged as amorphous
carbon.
At 500°C the decomposition was not sufficient to develop
highly porous membrane structure and the newly-developed
pores were constantly decreased in size due to the thermal
shrinkage. The decomposition to thermal shrinkage ratio
might have increased significantly at pyrolysis temperature of
600°C as suggested by the substantial increases of the gas
permeabilities. The decomposition rate reduced as the
decomposable structure of the membrane not much left, resulting the dominance of structural densification when the
pyrolysis temperature was further increased to 700°C. The
pores of carbon membrane might have collapsed and given
away for the initiation of carbon graphitization which had
higher density, tighter packaging and higher ordered structure
[9].
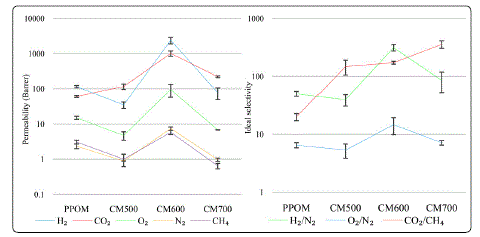
Figure 2: Permeabilities and ideal selectivities of PPO (PPOM) and PPO-derived hollow fiber carbon membrane (CM500, CM600 and CM700) prepared at different pyrolysis temperatures
The slightly higher CO2 permeabilities than their H2
counterparts as shown by the CM500 and CM700 indicated
the presence of surface diffusion as one of the contributing
transport mechanism besides molecular sieving effect. The
lower H2 permeability on both membranes indicated that the
resistance into the membrane was high most probably due to
smaller openings than that of CM600. At the same time, this
pore resistance also accelerated the accumulation of the
highly adsorbable CO2 at the narrow opening which eventually
provided suitable environment to produce surface diffusion
effect that increased the CO2 permeability.
The affinity between the CO2 and carbon membrane pore
wall suggested that kinetic diameter was not the sole factor in
determining the magnitude of the gas permeabilities, but also
the affinity of the diffusing molecules towards the carbon
membrane pore wall [10]. This affinity is originated from the van der Waal's forces produced by quadrupole moment of
the symmetrical CO2 that established weak attraction towards
the carbon pore walls which occupied with delocalized
electrons from its turbostratic structure.
Conclusion
The morphology of the PPO-derived hollow fiber carbon membrane was dense, homogenous, thin and symmetric. Each stage of pyrolysis temperature has significant impact on the PPO-derived carbon membrane structures. The highest permeabilities and ideal selectivities were obtained at 600°C. In exception of CO2 permeability and CO2/CH4 ideal selectivity, all permeabilities and ideal selectivities were very low when pyrolysis temperature of 500°C was used. The transport mechanism through the carbon membranes was highly affected by molecular sieving and surface diffusion. The surface diffusion transport mechanism was highly dominating when the carbon membranes possessed very small pore sizes.
Acknowledgements
The authors are grateful for the funding from Solid Waste Management Cluster (1001/CKT/870023), Membrane Science and Technology Cluster (1001/PSF/8610011), Postgraduate Research grant of Universiti Sains Malaysia and Mybrain 15 fellowship of Ministry of Higher Education, Malaysia.
References
- Barsema JN, Balster J, Jordan V, Van der Vegt NF, Wessling M. Functionalized carbon molecular sieve membranes containing Agnanoclusters. J Membrane Sci. 2003; 219(1-2): 47-57. doi: 10.1016/ S0376-7388(03)00176-5
- Tanco M, Tanaka D, Rodrigues S, Texeira M, Mendes A. Compositealumina- carbon molecular sieve membranes prepared from novolac resin and boehmite. Part I: Preparation, characterization and gas permeation studies. Int J Hydrogen Energy. 2015; 40(16): 5653-5663. doi: 10.1016/j.ijhydene.2015.02.112
- He X, Hagg M. Structural, kinetic and performance characterization of hollow fiber carbon membranes. J Membrane Sci. 2012; 390-391: 23-31. doi: 10.1016/j.memsci.2011.10.052
- Zhong Z, Yao J, Low Z, Chen R, He M, Wang H. Carbon composite membrane derived from a two-dimensional zeolitic imidazolate framework and its gas separation properties. Carbon. 2014; 72: 242-249. doi: 10.1016/j.carbon.2014.01.072
- Yoshimune M, Fujiwara I, Suda H, Haraya K. Novel carbon molecular sieve membranes derived from poly(phenylene oxide) and its derivatives for gas separation. Chem Lett. 2005; 34: 958-959. doi: 10.1246/ cl.2005.958
- He X, Lie J, Sheridan E, Hagg M. Preparation and characterization of hollow fiber carbon membranes from cellulose acetate precursors. Ind. Eng. Chem. Res. 2011; 50(4): 2080-2087. doi: 10.1021/ie101978q
- Shelekhin AB, Dixon AG, MaYH. Theory of gas diffusion and permeation in inorganic molecular-sieve membranes. AIChe J. 1995; 41(1): 58-67. doi: 10.1002/aic.690410107
- Chen X, Khoo KG, Kim MW, Hong L. Deriving a CO2-permselective carbon membrane from a multi-layered matrix of polyion complexes. ACS Appl Mater Inter. 2014; 6(13): 10220-10230. doi: 10.1021/am5015953
- Meng L. Park S. Investigation of narrow pore size distribution on carbon dioxide capture of nanoporous carbons. Bull Korean Chem Soc. 2012; 33(11): 3749-3754.



