Research Article
Effect of different solvents and clay loading on Polyacrylamide hydrogel to remove Iron from Lean Methyldiethanol solvent
1Department of Chemical Engineering, Khalifa University of Science and Technology, Abu Dhabi, United Arab Emirates
2Department of Chemistry, Khalifa University of Science and Technology, Abu Dhabi, United Arab Emirates
*Corresponding author: Fawzi Banat, Professor, Department of Chemical Engineering, Khalifa University of Science and Technology, Abu Dhabi, United Arab Emirates, E-mail: fbanat@pi.ac.ae
Received: March 9, 2017 Accepted: March 22, 2017 Published: March 27, 2017
Citation: Pal P, Gaber S, Haija MA, Banat F. Effect of different solvents and clay loading on Polyacrylamide hydrogel to remove Iron from Lean Methyldiethanol solvent. Int J Petrochem Res. 2017; 1(1): 6-11. doi: 10.18689/ijpr-1000102
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The polyacrylamide (PAAM) hydrogel consisting of acrylamide and 2(methacryloyloxy) ethyl aceto acetate as monomer, N, N/-methylenebisacrylamide as crosslinker and
ammonium peroxodisulfate as initiator was prepared using different solvents. Methanol, ethanol, dimethylsulfoxide and deionized water was used as solvent to prepare the
hydrogel. Its usefulness as adsorbent for the removal of iron as contaminants from
industrial lean methyl diethanol amine solvents (MDEA, 50 weight % used by GASCO
Company, Abu Dhabi for natural gas sweetening) was tested. Finally, PAAM hydrogel in
water with nitric acid treated sepiolite having different loading were prepared to increase
the mechanical strength of the composite hydrogel. SEM and FTIR analysis of the PAAM
clay composite hydrogel explained the morphology and adsorption process. PAAM
composite hydrogel with 12.5 % clay loading was found to be best using batch adsorption
studies for the removal of iron (45.15% having uptake capacity 6.397 µg/g using 1.0 g
hydrogel) from industrial lean MDEA solvent.
Keywords: Gas sweetening; hydrogel; effect of solvent; clay loading; adsorption
Introduction
GASCO Company, Abu Dhabi is currently using methyldiethanolamine (MDEA, 50
weight %) as solvent to remove H2S/CO2 from natural gas. In this process, reaction
between aerial oxygen and H2S/CO2with MDEA produces low molecular weight organic
acid anions such as formate, acetate, propionate etc. These anionic species and
protonated MDEA makes heat stable salt (HSS) which is not possible to be separated
from the solvents in the regenerator [1] [2]. Accumulation of HSS continuously in the
lean solvent deteriorates its quality making less sensitive for absorption. The increase in
HSS also leads to foaming in the process as well as corrosion and fouling of the process
equipment [3] [4] [5]. To maintain 50 weight % MDEA solution, make-up water as well
as fresh MDEA are added frequently in the process. Also, heavy metal ions such as iron, chromium, lead etc.is another class of contaminants found in lean MDEA solvents. It is
coming mostly due to metal corrosion and erosion in the process. The heavy metal ions
present in lean MDEA samples was mainly iron ranging from 900-1500 µg/g as found in
different batches [6]. Accumulation of iron as metal ions due to corrosion in running
plant enhance foaming issues [7]. Iron complexing with MDEA degradation products
also causes corrosion [8]. Again, iron produces soluble complex in alkanolamine solution
saturated with carbon dioxide supports dissolution of steel surfaces [9]. Foaming
tendency was increased by addition of 0.235 weight % ferrous sulfide on 50 weight %MDEA [10]. The functional groups in MDEA (tertiary amine
and hydroxyl) is easily attached with heavy metal ions using
chelation. Thus, adsorbent like activated carbon cannot
remove heavy metal ions overcoming this complexation. Currently, GASCO Company is using commercially available
activated carbon as adsorbent to remove mostly HSS anions. Few attempts were tried to remove HSS anions and metal ions
from alkanolamine solutions using distillation and electrodialysis
[11] [12] [13]. These processes suffer serious setbacks
in utility requirements in terms of continuous supply of huge
volumes of waterand no more a cost efficient process
From last decade, polymeric hydrogel showed superior
quality in removing different types of inorganic (such as
chromium, lead, cobalt etc.) as well as organic pollutants
using adsorption [14] [15]. The adsorption mainly results from
the presence of large number of functional groups remain in
the polymeric hydrogel network [16]. The presence of specific
functional groups (such as -OH, -NH2, -SO3H, -COOH, -CONH2
etc.) in hydrogel networks enables adsorptive removal of
pollutants [17]. In recent studies, it was observed that
hydrogels have superiority over conventional adsorbents like
carbon in terms of adsorption capacity [18]. Hydrogels are
similar to ion-exchange resins in many respects; for instance, they are polymeric materials and mostly remove pollutants
through electrostatic interactions. However, resins have rigid
structures while the structures of hydrogels are flexible and
can imbibe much more pollutants compared to resins [19]. The pollutants can be adsorbed onto the outer surface as well
as in the swollen three dimensional network of hydrogels. It
has been shown that hydrogel properties can be varied by
controlling the synthesis parameters, such as amount of
monomers reaction time, temperature, and solvent used to
prepare hydrogels [20]. Advantages of using hydrogel as
adsorbents are due to its easy loading, reusability and the
possibility of continuous operation [21] [22]. High swelling and wettability of the hydrogel also facilitates adsorption as
the swelling of the three-dimensional networks allots high
surface area and more exposed functional groups easily
approachable for adsorption [23]. Hydrogels are generally
prepared by copolymerization and crosslinking of functional
monomers. Solvent has enormous role while producing
polymeric hydrogels in terms of structure and morphology of
the three dimensional network [24]. Even, mixed solvents like
water and tetrahydrofuran were used to modulate
polymerization and cross-linking of N-isopropylacrylamide to
obtain useful three dimensional hydrogel structure [25]. Most
of the works using hydrogel beads as adsorbents usually focus
on physicochemical and textural characterization, but very
few has dealt with the mechanical properties of the hydrogels. These properties are relevant in order to select the type of
equipment and the best operating conditions to achieve
good adsorption performance at full-scale.
Hydrogel as adsorbents should be able to sustain the load
they will be subjected to during handling and adsorption; consequently, an important issue is to determine if deformation
affects their use, by reducing the voidage of flattened beads or increasing the resistance of the flow of liquids around them
[26]. However, few studies have taken into account the presence
of fillers in the hydrogel matrix affects the final characteristics
of the beads. The latter is particularly important for their further
commercialization as adsorbents. Moreover, mechanical
strength of polymeric beads remains almost unexplored. Recently, the compression strength of alginate hydrogel
cylinders reinforced with GO to the alginate matrix has been
studied [27]. Mostly, clay composite hydrogels show good
mechanical strength and used effectively as adsorbents for the
removal of contaminants from solutions [28]. Sepiolite is one of
the easily available low cost clay is being used as adsorbent for
long for the removal of metal ions from solutions [29]. In our
previous studies, removal of heavy metal ions like iron and
chromium from MDEA solutions using polyacrylamide hydrogel (PAAM) was explored [30]. Though good removal was achieved, the polymeric PAAM adsorbents did not show good mechanical
strength urged for more useful alternatives. To the best of our
knowledge, no study has yet reported mechanically stable
polyacrylamide sepiolite composite hydrogel beads for the
removal of iron from aqueous lean MDEA solutions.
Aim of the work
The major aim of this research work is to explore the possible use of polyacrylamide (PAAM) hydrogel as effective adsorbent for the effective removal of iron from industrial lean MDEA solutions. Solvent plays a major role in polymerizing and cross linking to produce effective three dimensional network hydrogel to be used as adsorbent. Thus, PAAM hydrogel was prepared using three different organic solvents such as methanol, ethanol, dimethyl sulfoxide including deionized water to select the best to be used as adsorbent. The mechanical strength of the hydrogel is another useful parameter to be effectively used as adsorbents. Sepiolite, is a well-know clay used in this study to be added to increase the mechanical strength of the PAAM hydrogel. Sepiolite clay was treated with 0.1 (N) nitric acid solution to avoid any metal leaching to the MDEA solution. The composite PAAM hydrogel using nitric acid treated sepiolite clay of different loading was prepared to increase the mechanical strength to be used as adsorbent. The best selected PAAM sepiolite composite hydrogel was characterized using FTIR and SEM analysis. Adsorption studies were conducted using PAAM hydrogels using different solvents as well as PAAM sepiolite composite hydrogels to find best possible hydrogel for the removal of iron from industrial lean MDEA solvent.
Materials and Methods
Materials and instrumentations
All the chemicals were purchased from and used without
any further purification. Chemicals used in this study are
obtained (acrylamide, C3H5NO; ammonium peroxodisulphate, (NH4)2S2O8; N,N'-methylenebisacrylamide, C7H10N2O2Merck, Germany/ 2(methacryloyloxy) ethyl aceto acetate, C10H14O5, Aldrich/ dimethyl sulfoxide dehydrated, C2H6OS; VWR Chemicals, BDH and methanol, CH3OH; ethanol, C2H5OH; Fisher Scientific) from different sources. MDEA solvent
containing 50 weight % MDEA was provided by GASCO (Habshan, Abu Dhabi) and sepioliteclay was purchased from
Sigma Aldrich, Germany.
Elemental analysis was carried out using Inductively
Coupled Plasma Optical Emission Spectrometry (ICP-OES, Optima 8000, Perkin Elmer). Scanning Electron Microscopy (SEM) was carried out with FEI Quanta 200, The Netherland. The FTIR studies of lean MDEA, PAAM sepiolite clay composite
before and after adsorption were carried out using FTIR, Nicolet, iS10, Thermo Scientific instrument using OMNIC
software (number of scans 64, resolution 4).
Methods
Preparationof hydrogel using different solvents
Hydrogel was prepared using acrylamide (AM) and
2-(methacryloyloxy) ethyl acetoacetate (AAEM) as monomer
using free radical polymerization. N, N-methylenebisacrylamide (MBA) was used as cross-linker and ammonium
peroxodisulphate (APS) was used as initiator. Methanol, ethanol, dimethyl sulfoxide (DMSO) and deionized water was
used as solvent. Acrylamide (300.0 mg) was dissolved in 2 mL
distilled water in 25 mL glass vials. 200 µL AAEM was dissolved
in 2 mL methanol/ethanol/DMSO/water was added to AM
solution. The mixture was stirred for 2 h to get homogenous
mixture. Then, 300 µL saturated MBA was added as cross
linker. Finally, 100 µL 10 weight% APS was added to initiate
polymerization reaction. The mixture was transferred into a
water bath at 60oC for 2 h and kept at room temperature for
24 h to ensure complete polymerization. The resulting
polymer hydrogel was cut into small pieces and immersed in
deionized water to remove any unreacted monomers. Finally, the hydrogel was placed in an oven at 45-50oC for 15 min
before use as adsorbent.
Preparation of polyacrylamide hydrogel using different
amount of sepiolite clay
Acrylamide (300.0 mg) was dissolved in 2 mL deionized
water. Predetermined amount of 0.1 (N) nitric acid treated
sepiolite clay was added and mixed to get homogeneous
solution. MBA (300 µL) and APS (100 µL) was added
subsequently to the acrylamide solution. The mixture was
transferred into a water bath at 60oC for 2 h followed by 24 h
at room temperature. The composite hydrogel was washed
with distilled water and oven dried for 15 minutes at 45-50°C
before use as adsorbent.
Analysis of iron in lean MDEA
The iron content in lean MDEA was determined by
Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, Optima 8000; Perkin Elmer) and found to be 1417
µg/g (). The argon flow rate was 12 L/min and air flow rate (1.2
L/min). Internal standards yttrium (1.0 ppm) was added to
correct the physical interferences of aqueous-organic mixture
on 2% nitric acid as blank, standards and MDEA solution [3].
Batch adsorption study
Iron adsorption from lean MDEA was conducted in batch
mode in 50 mL stoppered conical flasks containing 1.0 g () of
adsorbent in 10 mL lean MDEA solution () at 25°C. The flasks
were placed on a water bath shaker (Dihan, Korea) at 140 rpm
for 24 h to attain equilibrium. The supernatants were extracted
and analyzed using ICP-OES for equilibrium concentration (). The amount of contaminants removed using batch
equilibrium study was calculated as:
qe Uptake capacity of iron adsorbed per unit mass of hydrogel
at equilibrium (µg/g) V = volume of lean MDEA (mL) M = mass of adsorbent (g)
Results and Discussions
Polyacrylamide hydrogels using different solvents
The PAAM hydrogels prepared using different solvents
were found to have different texture. The produced hydrogels
are shown in Figure 1. Hydrogels prepared from methanol, ethanol, DMSO and water were cut into pieces and used for
adsorption studies. PAAM hydrogel prepared using methanol
was colorless. While, hydrogel prepared using ethanol was
found to be milky with good textural appearance and found
to be less swelled in lean MDEA. However, it was unstable and
became softer when added to lean MDEA solvent. Hydrogel
produced using DMSO solvent was found to be fast gelling
during polymerization and yellowish in color and best in
mechanical strength and less swelling. While, PAAM hydrogel
prepared using water was best to use as less corrosive as
compare to other organic solvents.
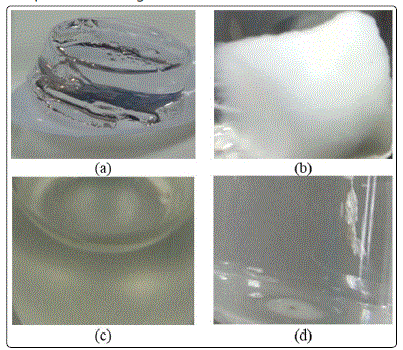
Figure 1: PAAM hydrogel prepared using(a) methanol,(b) ethanol, (c) DMSO and (d) water as solvent.
Polyacrylamide hydrogels with different clay loading
Composite PAAM hydrogel using 0.1 (N) nitric acid
treated sepiolite clay having different amount are shown in
Figure 2. It was observed that composite hydrogels were
denser with increasing clay loading.
Figure 2: PAAM clay composite hydrogel prepared using (a) 3.125 % clay, (b) 6.25 % clay, (c) 9.375 % clay and (d) 12.5 % clay.
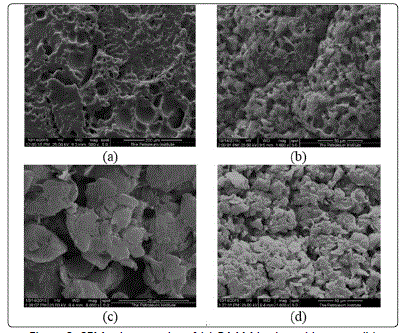
SEM analysis
The PAAM composite hydrogel beads with different weight
% of sepiolite clay were observed using Scanning Electron
Microscopy (SEM) to understand surface properties. The SEM
images are shown in Figure 3.Composite PAAM hydrogel with
different amount of sepiolite was found to be heterogeneous
with internal cavity. As the amount clay increased in polyacrylamide
matrix, the surface morphology was changed. The surface
became more homogeneous near to outer surface, while at the
center of the bead it was found to be more compact. In Figure
3 (b), it can be seen that there is well-distributed porosity at the
center. At higher clay loadings, Figures 3 (c and d) the center of
the beads became denser and compact, and there was no
distinct improvement in surface porosity.
Figure 3: SEM micrographs of (a) PAAM hydrogel in water (b) composite PAAM hydrogel 3.125% sepiolite (c) PAAM-9.375% sepiolite(d)PAAM-9.375% sepiolite.
FTIR analysis
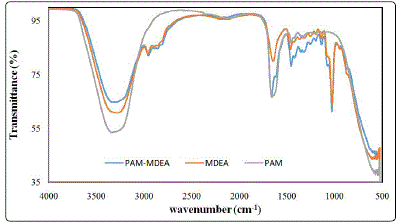
Figure 4 shows the FTIR spectra of lean MDEA and PAAM
sepiolite clay composite hydrogel before and after adsorption. The composite hydrogel spectrum showed a peak at 1020
cm-1 in addition to PAAM characteristic peaks [13]. The peak
represents Si-OH coming from sepiolite clay. In PAAM
hydrogel spectrum, the broad peaks centered at 3295 cm-1
represents OH stretching vibration resulted from the residual
water molecules in the hydrogel. Also, NH2 stretching vibration
at the same region increased the intensity of the peak. The
peak at 1650 cm-1 is attributed to C=O stretching. The peaks
at 1412 and 1446 cm-1 are due to stretching vibrations of the
C-N bond and CH2 bending vibrations, respectively. While, in
lean MDEA and in adsorbed hydrogel sample similar peaks
were determined. After adsorption, new IR peak appear at
1451 cm-1 indicates the adsorption of iron on PAAM composite
hydrogel surface [31].
Figure 4: FTIR spectra of MDEA and polyacrylamide hydrogel before and after adsorption in MDEA
Adsorption studies
Polyacrylamide hydrogel using different solvents
The uptake capacity and % removal of iron on PAAM
hydrogel prepared using different solvents are tested to
remove iron from lean MDEA as shown in Figure 5. Metanol (1), ethanol (2), DMSO (3) and deionized water (4) was used in
this study. The % iron removal as well as uptake capacity using
methanol solvent to prepare PAAM hydrogel was found to be
23.19 % and3.286 µg/g, respectively. The removal efficiency
was found almost similar using DMSO solvent (21.0 % iron
removal having uptake capacity of 2.975 µg/g). While, PAAM
hydrogel prepared using ethanol gave the lowest iron removal (14.21 %) and subsequently minimum uptake capacity (2.013µg/g). PAAM hydrogel prepared using water was found
to be best for the removal of iron (24.75 % having uptake
capacity 3.286 µg/g) from lean MDEA [30]. Therefore, it was
used further to prepare composite hydrogel for the adsorption
of iron from lean MDEA solvent.
Figure 5: Effect of different solvent(1 methanol; 2 ethanol; 3 dimethyl sulfoxide and 4 deionized water) on uptake capacity and % removal of iron using 1.0 g of each PAAM hydrogel in 10 mL lean MDEA for 24 h at 298K.
Polyacrylamide composite hydrogel using different clay
loading
To increase the mechanical strength of the hydrogel
PAAM-nitric acid treated sepiolite clay composite was used. It
was evident that sepiolite can be effectively used as adsorbent
to remove metal ions from solutions and with increasing
amount of sepiolite in solution decreases the equilibrium
metal ions concentrations [32]. Thus, uptake capacity as well
as % removal should be higher with increasing sepiolite
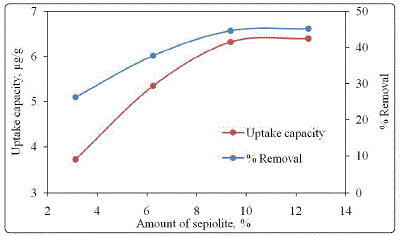
loading. The uptake capacity and % removal of iron on
increasing amount of sepiolite loaded PAAM hydrogels is
shown in Figure 6. It was observed that both % iron removal
as well as uptake capacity was increased with increasing clay
content and remain almost constant with 12.5 % of sepiolite
clay loading. The % iron removal was increased from 26.25-
45.15 % with sepiolite loading increased from 3.125-12.5
weight %. The uptake capacity was also found to be increased
from 3.125-6.397 µg/g with increase in sepiloite loading on
PAAM hydrogel matrix.
Figure 6: Effect of clay loading on uptake capacity and % removal of iron using 1.0 g of each PAAM- sepiolite composite hydrogel in 10 mL lean MDEA for 24 h at 298K.
Conclusion
This research work investigated the preparation of PAAM hydrogel using different solvent and used as an adsorbent for the removal of iron from industrial lean MDEA. Among methanol/ethanol/DMSO/water used, PAAM hydrogel prepared using water was found to be best for the removal of iron (24.75 % having uptake capacity 3.50µg/g using 1.0 g PAAM hydrogel) from lean MDEA solvent. SEM and FTIR analysis were carried out using 0.1 (N) nitric acid treated sepiolite composite PAAM to characterize the hydrogel. The 12.5 % loaded sepiolite PAAM composite hydrogel exhibited the highest percentage removal of iron from industrial lean MDEA solvent.
Acknowledgement
The authors are grateful to the Gas Research Centre, The Petroleum Institute, Abu Dhabi for funding the project (GRC 006). Sincere thanks to GASCO Company (Habshan, Abu Dhabi) for their co-operation and support.
References
- Mandal BP, Biswas AK, Bandopadhyay SS. Selective adsorption of H2S from gas streams containing H2S and CO2 into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol. Sep. Puri. Tech. 2004; 35(3): 191-202. doi: 10.1016/S1383-5866(03)00139-4
- Lu JG, Zheng YF, He DL. Selective absorption of H2S from gas mixtures into aqueous solution of blended amines of methyldiethanolamine and 2-tertiarybutylamino-2-ethoxyethanol in a packed column. Sep. Puri. Tech. 2006; 52(2): 209-217. doi: 10.1016/j.seppur.2006.04.003
- Nielsen RB, Lewis KR. Corrosion in Refinery Amine Systems, Corrosion/95; NACE International Houston. 1995; 571.
- Rooney PC, DuPart MS, Bacon TR. Effect of heat stable salts on MDEA solution corrosivity, Hydro. Proc. 1997; 76: 65-68.
- Keller AE, Kammiller RM, Veatch FC, Cummings AL, Thompsen JC, Mecum SM. Heat-stable salt removal from amines by the HSSX process using ion exchange. Proceedings of 42nd Annual Laurance Reid Gas Conditioning Conference. The University of Oklahoma OK. 1992; 61-92.
- Pal P, AbuKashabeh A, Al-Asheh S, Banat F. Role of aqueous methyldiethanolamine (MDEA) as solvent in natural gas sweetening unit and process contaminants with probable reaction pathway. J. Nat. Gas Sci. Eng. 2015; 24: 124-131. doi: 10.1016/j.jngse.2015.03.007
- Pal P, Banat F. Comparison of heavy metal ions removal from industrial lean amine solvent using ion exchange resins and sand coated with chitosan. J. Nat. Gas Sci. Eng. 2014; 18: 227-236. doi: 10.1016/j.jngse.2014.02.015
- Pal P, Banat F. Comparison of thermal degradation between fresh and industrial aqueous methyldiethanolamine with continuous injection of H2S/CO2 in high pressure reactor. J. Nat. Gas Sci. Eng. 2016; 29: 479-487. doi: 10.1016/j.jngse.2016.01.037
- Ross TK, Pearson C. The corrosion of mild steel by ethanolamine solutions. Corro. Sci. 1964; 4(1-4): 449-452. doi: 10.1016/0010-938X(64)90046-0
- Alhseinat E, Pal P, Keewan M, Banat F. Foaming study combined with physical characterization of aqueous MDEA gas sweetening solutions. J. Nat. Gas Sci. Eng. 2014; 17: 49-57. doi: 10.1016/j.jngse.2013.12.004
- Meng H, Li H, Li CX. Synthesis of ionic liquid using a four-compartment configuration electrodialyzer. J. Mem. Sci. 2008; 318(1-2): 1-4. doi: 10.1016/j.memsci.2008.02.020
- Cheryan M, Parekh SR. Separation of glycerol and organic acids in model ethanol stillage electrodialysis and precipitation. Proc. Biochem. 1995; 30: 17-23. doi: 10.1016/0032-9592(95)87003-2
- Hong M, Shuang Z, Chunxi L, Liangshi L. Removal of heat stable salts from aqueous solutions of N-methyldiethanolamine using a specially designed three-compartment configuration electrodialyzer. J. Mem. Sci. 2008; 322(2): 436-440. doi: 10.1016/j.memsci.2008.05.072
- Tang SCN, Lo IMC, Mak MSH. Comparative Study of the adsorptionselectivity of Cr (VI) onto cationic hydrogels with different functional groups. Wat. Air Soil Pollu. 2012; 223(4): 1713-1722. doi: 10.1007/s11270-011-0977-4
- Khan M, Lo IMC. Removal of ionizable aromatic pollutants from contaminated water using nanoγ-Fe2O3 based magnetic cationic hydrogel: sorptive performance, magnetic separation and reusability. J. Hazard. Mater. 2017; 322: 195-204. doi: 10.1016/j.jhazmat.2016.01.051
- Merino S, Martín C, Kostarelos K, Prato M, Vazquez E. Nanocomposite hydrogels: 3D polymer-nanoparticle synergies for on-demand drug delivery. ACS Nano. 2015; 9(5): 4686-4697. doi: 10.1021/acsnano.5b01433
- Ozay O, Ekici S, Baran Y, Aktas N, Sahiner N. Removal of toxic metal ions with magnetic hydrogels. Wat. Res. 2009; 43(17): 4403-4411. doi: 10.1016/j.watres.2009.06.058
- Zhu Y, Zheng Y, Wang F, Wang A. Monolithic super macroporous hydrogel prepared from high internal phase emulsions (HIPEs) for fast removal of Cu2+and Pb2+. Chem. Eng. J. 2016; 284: 422-430.
- Tang SCN, Wang P, Yin K, Lo IMC. Synthesis and application of magnetichydrogel for Cr (VI) removal from contaminated water. Environ. Eng. Sci. 2010; 27(11): 947-954. doi: 10.1089/ees.2010.0112
- Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 2015; 6(2): 105-121. doi: 10.1016/j.jare.2013.07.006
- Bekiari V, Sotiropoulou M, Bokias G, Lianos P. Use of poly(N,N dimethylacrylamide-co-sodium acrylate) hydrogel to extract cationic dyes and metals from water. Colloids Surf.A. 2008; 312: 214-218.
- Kasgoz H, Ozgumus S, Orbay M. Modified polyacrylamide hydrogels and their application in removal of heavy metal ions. Poly. 2003; 44(6): 1785–1793. doi: 10.1016/S0032-3861(03)00033-8
- Trakulsujaritchok T, Noiphom N, Tangtreamjitmun N, Saeeng R. Adsorptive features of poly(glycidylmethacrylateco-hydroxyethyl methacrylate): effect of porogen formulation on heavy metal ion adsorption. J. Mater. Sci. 2011; 46(16): 5350-5362. doi: 10.1007/s10853-011-5473-
- Raeburn J, Mendoza CC, Cattoz BN, Little MA, Terry AE, Cardoso AZ, Griffiths PC, Adams DJ. The effect of solvent choice on the gelation and final hydrogel properties of Fmoc-diphenylalanine. Soft Mat. 2015; 11: 927-935. doi: 10.1039/C4SM02256D
- Zhang X, Yang Y, Chung T. Effect of mixed solvents on characteristics of poly(N-isopropylacrylamide) gels. Lang. 2002; 18 (7): 2538-2542. doi: 10.1021/la011410j
- Chiew CSC, Yeoh HK, Pasbakhsh P, Krishnaiah K, Poh PE, Tey BT, Chan ES. Halloysite/alginate nanocomposite beads: kinetics, equilibrium and mechanism for lead adsorption. Appl. Clay. Sci. 2016; 119: 301-310. doi: 10.1016/j.clay.2015.10.032
- Zhang H, Pang X, Qi Y. pH-Sensitive graphene oxide/sodium alginate/ polyacrylamide nanocomposite semi-IPN hydrogel with improved mechanical strength. RSC Adv. 2015; 5: 89083-89091. doi: 10.1039/ C5RA19637J
- Khan M, Lo IMC. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016; 106: 259-271. doi: 10.1016/j. watres.2016.10.008
- Padilla-Ortega E, Leyva-Ramos R, Mendoza-Barron J, Guerrero- Coronado RMA, Jacobo-Azuara A, Aragon-Pina A. Adsorption of Heavy Metal Ions from Aqueous Solution onto Sepiolite. Ads. Sci. Tec. 2011; 29(6): 569-584. doi: 10.1260/0263-6174.29.6.569
- Pal P, Banat F. Removal of contaminants from industrial lean amine solvent using polyacrylamide hydrogels optimized by response surface methodology. Ads. Sci. Tec. 2015; 33(1): 9-24. doi: 10.1260/0263- 6174.33.1.9
- Tandon PK, Shukla RC, Singh SB. Removal of arsenic (III) from water with clay-supported zerovalent iron nanoparticles synthesized with the help of tea liquor. Ind. Eng. Chem. Res. 2013; 52(30): 10052-10058. doi: 10.1021/ie400702k
- Kara M, Yuzer H, Sabah E, Celik MS. Adsorption of cobalt from aqueous solutions onto sepiolite. Wat. Res. 2003; 37(1): 224-232. doi: 10.1016/ S0043-1354(02)00265-8